Page 231 - Book Hosokawa Nanoparticle Technology Handbook
P. 231
4.4 NANOCOMPOSITE STRUCTURE FUNDAMENTALS
be summarized as (1) transport of oxide ions from the claimed that the experimental data are satisfactorily
electrolyte through the ionic conductors and transport fitted by setting the coordination number z 1.764
i/i
of reactants from the anode surface to the active sites (i denotes electronic or ionic conductor) at the perco-
through the pores; (2) electrochemical reaction at the lation threshold, below which no electronic or ionic
active sites; and (3) transport of electrons from the conducting cluster connects to both ends of elec-
active sites to the current collector through the elec- trode. The percolation probability that an arbitrary
tronic conductors and transport of products from the particle belongs to a percolation cluster is given as
active sites to the anode surface through the pores.
Since the electrode performance is highly depend- ⎧ 2.5 ⎫ 0.4
.
ent on its microstructure, the anode electrode must be p 1 ⎨ ⎪ ⎛ ⎜ 4 236 z ⎞ ⎟ ⎪ ⎬ (4.4.1)
ii
designed as a homogeneous or graded structure con- i ⎩ ⎪ ⎝ 2 472 ⎠ ⎭ ⎪
.
sisting of three phases, providing percolation paths
for electrons, oxide ions, and gaseous hydrogen and
water, respectively. An analogous framework holds The coordination number of electronic to electronic
for the cathode electrode in which oxide ions are gen- conductors, z el/el , and that of ionic to ionic conductors,
erated via reduction of oxygen molecules. z io/io , are
zn
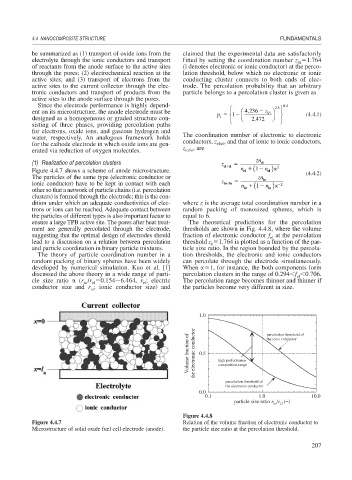
(1) Realization of percolation clusters z el el n 1 ( el 2
Figure 4.4.7 shows a scheme of anode microstructure. el n ) (4.4.2)
el
The particles of the same type (electronic conductor or zn io
ionic conductor) have to be kept in contact with each z io io n 1 ( n ) 2
other so that a network of particle chains (i.e. percolation io io
clusters) is formed through the electrode; this is the con-
dition under which an adequate conductivities of elec- where z is the average total coordination number in a
trons or ions can be reached. Adequate contact between random packing of monosized spheres, which is
the particles of different types is also important factor to equal to 6.
ensure a large TPB active site. The pores after heat treat- The theoretical predictions for the percolation
ment are generally percolated through the electrode, thresholds are shown in Fig. 4.4.8, where the volume
suggesting that the optimal design of electrodes should fraction of electronic conductor f at the percolation
el
lead to a discussion on a relation between percolation threshold z 1.764 is plotted as a function of the par-
ii
and particle coordination in binary particle mixtures. ticle size ratio. In the region bounded by the percola-
The theory of particle coordination number in a tion thresholds, the electronic and ionic conductors
random packing of binary spheres have been widely can percolate through the electrode simultaneously.
developed by numerical simulation. Kuo et al. [1] When 1, for instance, the both components form
discussed the above theory in a wide range of parti- percolation clusters in the range of 0.294 f 0.706.
el
cle size ratio (r /r 0.154 6.464, r ; electric The percolation range becomes thinner and thinner if
el
el
io
conductor size and r ; ionic conductor size) and the particles become very different in size.
io
1.0
Volume fraction of the electronic conductor 0.5 high performance percolation threshold of
the ionic conductor
composition range
percolation threshold of
the electronic conductor
0.0
0.1 1.0 10.0
particle size ratio r /r (−)
io el
Figure 4.4.8
Figure 4.4.7 Relation of the volume fraction of electronic conductor to
Microstructure of solid oxide fuel cell electrode (anode). the particle size ratio at the percolation threshold.
207