Page 228 - Book Hosokawa Nanoparticle Technology Handbook
P. 228
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
target pH swing value (for instance, the pH at both The specific surface area of alumina decreases
ends is from 2 to 10), the raw material solution of the remarkably through the transformation into alu-
obtained amount is added from the titration curve. mina. It is thought as a main current before Schaper’s
The precipitation of pseudo-boehmite is formed in the paper [4] that the decrease of specific surface area at
neutral area of the pH. A small pseudo-boehmite crys- the high temperature of alumina is caused by the re-
tallite dissolves and disappears because the pH at both arrangement from the cubic arrangement of the oxy-
ends is the dissolution areas of pseudo-boehmite. gen ion into the hexagonal one. Schaper et al.
Relatively large pseudo-boehmite that remains with- reported that sintering is a phenomenon of alumina
out dissolving becomes a nucleus, and pseudo- itself, and the phase transition to alumina is caused
boehmite grows up in the following pH swing as a result. It is known that some alumina shows a
operation. Only a limited number of particles from large decrease of specific surface area at much lower
initial stage of synthesis can grow up by this operation temperature than the temperature of transformation of
because the nucleus newly generated disappears. The alumina to alumina. It proves the correctness of
pH swing operation is repeated several times until this idea. Afterwards, Burtin et al. [5] proposed the
pseudo-boehmite grows up and reaches a target pore model that the nucleus of alumina is generated at
size. As a result, the pseudo-boehmite crystallite the moment that the oxygen vacancy of alumina
diameter grows up with the pH swing operation fre- meets cation in the sintering process of alumina.
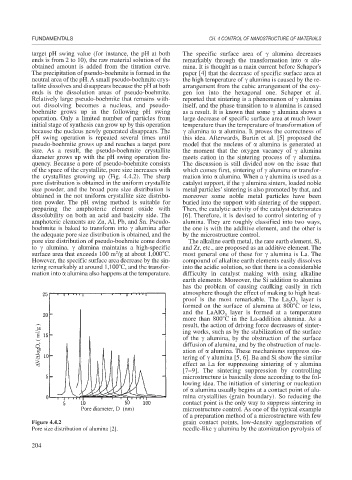
quency. Because a pore of pseudo-boehmite consists The discussion is still divided now on the issue that
of the space of the crystallite, pore size increases with which comes first, sintering of alumina or transfor-
the crystallites growing up (Fig. 4.4.2). The sharp mation into alumina. When a alumina is used as a
pore distribution is obtained in the uniform crystallite catalyst support, if the alumina sinters, loaded noble
size powder, and the broad pore size distribution is metal particles’sintering is also promoted by that, and
obtained in the not uniform crystallite size distribu- moreover some noble metal particles have been
tion powder. The pH swing method is suitable for buried into the support with sintering of the support.
preparing the amphoteric element oxide with Then, the catalytic activity of the catalyst deteriorates
dissolubility on both an acid and basicity side. The [6]. Therefore, it is devised to control sintering of
amphoteric elements are Zn, Al, Pb, and Sn. Pseudo- alumina. They are roughly classified into two ways,
boehmite is baked to transform into alumina after the one is with the additive element, and the other is
the adequate pore size distribution is obtained, and the by the microstructure control.
pore size distribution of pseudo-boehmite come down The alkaline earth metal, the rare earth element, Si,
to alumina. alumina maintains a high-specific and Zr, etc., are proposed as an additive element. The
2
surface area that exceeds 100 m /g at about 1,000 C. most general one of these for alumina is La. The
However, the specific surface area decrease by the sin- compound of alkaline earth elements easily dissolves
tering remarkably at around 1,100 C, and the transfor- into the acidic solution, so that there is a considerable
mation into alumina also happens at the temperature. difficulty in catalyst making with using alkaline
earth elements. Moreover, the Si addition to alumina
has the problem of causing caulking easily in rich
atmosphere though the effect of making to high heat-
proof is the most remarkable. The La O layer is
3
2
formed on the surface of alumina at 800 C or less,
and the LaAlO layer is formed at a temperature
3
more than 800 C in the La-addition alumina. As a
result, the action of driving force decreases of sinter-
ing works, such as by the stabilization of the surface
of the alumina, by the obstruction of the surface
diffusion of alumina, and by the obstruction of nucle-
ation of alumina. These mechanisms suppress sin-
tering of alumina [5, 6]. Ba and Si show the similar
effect as La for suppressing sintering of alumina
[7–9]. The sintering suppression by controlling
microstructure is basically done according to the fol-
lowing idea. The initiation of sintering or nucleation
of alumina usually begins at a contact point of alu-
mina crystallites (grain boundary). So reducing the
contact point is the only way to suppress sintering in
microstructure control. As one of the typical example
of a preparation method of a microstructure with few
Figure 4.4.2 grain contact points, low-density agglomeration of
Pore size distribution of alumina [2]. needle-like alumina by the atomization pyrolysis of
204