Page 520 - Book Hosokawa Nanoparticle Technology Handbook
P. 520
APPLICATIONS 18 DEVELOPMENT OF HIGH-PERFORMANCE ELECTROCHEMICAL REACTORS
80
t=600°C oxygen 2%
oxygen 2% 1000ppm-NO
oxygen 2% 1000ppm-NO
ox y y g g e e n n 2 % 1 1 0 0 0 0 0 0 p p p p m - - N O t=700°C
ox
N
O
m
2
%
t t=700
70 EC electrode e e 1000ppm-NO
e
el
c
e
EC electrode
EC
el
EC
o
o
d
d
r
t
c
r
t
Energy Pt-1300°C
Ag-800°C
NO x decomposition (%) 50 control system Literature
6 60
ordinary
Pd-800°C
nano-
catalyst
Pd-1300°C
scale
Pd-Pt-1300°C
meso-
scale
control
40
30
previous
20
results Cell stack
0
(100 × 100 × 50 mm)
10
0
0 50 100 150 200 250
Cell current (mA)
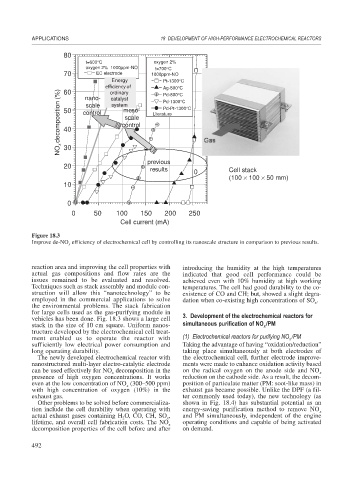
Figure 18.3
Improve de-NO efficiency of electrochemical cell by controlling its nanoscale structure in comparison to previous results.
x
reaction area and improving the cell properties with introducing the humidity at the high temperatures
actual gas compositions and flow rates are the indicated that good cell performance could be
issues remained to be evaluated and resolved. achieved even with 10% humidity at high working
Techniques such as stack assembly and module con- temperatures. The cell had good durability to the co-
struction will allow this “nanotechnology” to be existence of CO and CH; but, showed a slight degra-
employed in the commercial applications to solve dation when co-existing high concentrations of SO .
x
the environmental problems. The stack fabrication
for large cells used as the gas-purifying module in
vehicles has been done. Fig. 18.3 shows a large cell 3. Development of the electrochemical reactors for
stack in the size of 10 cm square. Uniform nanos- simultaneous purification of NO /PM
x
tructure developed by the electrochemical cell treat-
ment enabled us to operate the reactor with (1) Electrochemical reactors for purifying NO /PM
x
sufficiently low electrical power consumption and Taking the advantage of having “oxidation/reduction”
long operating durability. taking place simultaneously at both electrodes of
The newly developed electrochemical reactor with the electrochemical cell, further electrode improve-
nanostructured multi-layer electro-catalytic electrode ments were made to enhance oxidation activity based
can be used effectively for NO decomposition in the on the radical oxygen on the anode side and NO x
x
presence of high oxygen concentrations. It works reduction on the cathode side. As a result, the decom-
even at the low concentration of NO (300–500 ppm) position of particulate matter (PM: soot-like mass) in
x
with high concentration of oxygen (10%) in the exhaust gas became possible. Unlike the DPF (a fil-
exhaust gas. ter commonly used today), the new technology (as
Other problems to be solved before commercializa- shown in Fig. 18.4) has substantial potential as an
tion include the cell durability when operating with energy-saving purification method to remove NO x
actual exhaust gases containing H O, CO, CH, SO , and PM simultaneously, independent of the engine
2
x
lifetime, and overall cell fabrication costs. The NO x operating conditions and capable of being activated
decomposition properties of the cell before and after on demand.
492