Page 525 - Book Hosokawa Nanoparticle Technology Handbook
P. 525
19 DENDRIMERS AND THEIR APPLICATION TO ORGANIC ELECTRONICS DEVICES APPLICATIONS
Recently, phosphorescent dyes have drawn much
attention as light-emitting materials because of their
high luminescence efficiency. In contrast with fluores-
cent dyes, the phosphorescent quantum efficiency can
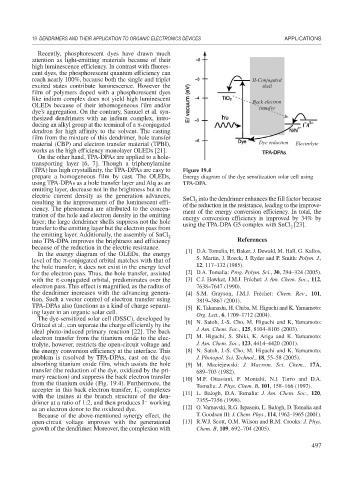
reach nearly 100%, because both the single and triplet II-Conjugated
excited states contribute luminescence. However the shell
film of polymers doped with a phosphorescent dyes
like indium complex does not yield high luminescent Back electron
OLEDs because of their inhomogeneous film and/or transfer
dye’s aggregation. On the contrary, Samuel et al. syn-
thesized dendrimers with an indium complex, intro-
ducing an alkyl group at the terminal of a -conjugated
dendron for high affinity to the solvent. The casting
film from the mixture of this dendrimer, hole transfer
material (CBP) and electron transfer material (TPBI), Dye reduction Electrolyte
works as the high efficiency monolayer OLEDs [21].
On the other hand, TPA-DPAs are applied to a hole-
transporting layer [6, 7]. Though a triphenylamine
(TPA) has high crystallinity, the TPA-DPAs are easy to Figure 19.4
prepare a homogeneous film by cast. The OLEDs, Energy diagram of the dye sensitization solar cell using
using TPA-DPAs as a hole transfer layer and Alq as an TPA-DPA.
emitting layer, decrease not in the brightness but in the
electric current density as the generation advances, SnCl into the dendrimer enhances the fill factor because
2
resulting in the improvement of the luminescent effi- of the reduction in the resistance, leading to the improve-
ciency. The phenomena are attributed to the concen- ment of the energy conversion efficiency. In total, the
tration of the hole and electron density in the emitting energy conversion efficiency is improved by 34% by
layer; the large dendrimer shells suppress not the hole using the TPA-DPA G5 complex with SnCl [23].
transfer to the emitting layer but the electron pass from 2
the emitting layer. Additionally, the assembly of SnCl 2
into TPA-DPA improves the brightness and efficiency References
because of the reduction in the electric resistance. [1] D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos,
In the energy diagram of the OLEDs, the energy
level of the -conjugated orbital matches with that of S. Martin, J. Roeck, J. Ryder and P. Smith: Polym. J.,
the hole transfer; it does not exist in the energy level 12, 117–132 (1985).
for the electron pass. Thus, the hole transfer, assisted [2] D.A. Tomalia: Prog. Polym. Sci., 30, 294–324 (2005).
with the -conjugated orbital, predominates over the [3] C.J. Hawker, J.M.J. Fréchet: J. Am. Chem. Soc., 112,
electron pass. This effect is magnified, as the radius of 7638–7647 (1990).
the dendrimer increases with the advancing genera- [4] S.M. Grayson, J.M.J. Fréchet: Chem. Rev., 101,
tion. Such a vector control of electron transfer using 3819–3867 (2001).
TPA-DPAs also functions as a kind of charge separat- [5] K. Takanashi, H. Chiba, M. Higuchi and K. Yamamoto:
ing layer in an organic solar cell. Org. Lett., 6, 1709–1712 (2004).
The dye-sensitized solar cell (DSSC), developed by
Grätzal et al., can separate the charge efficiently by the [6] N. Satoh, J.-S. Cho, M. Higuchi and K, Yamamoto:
ideal photo-induced primary reaction [22]. The back J. Am. Chem. Soc., 125, 8104–8105 (2003).
electron transfer from the titanium oxide to the elec- [7] M. Higuchi, S. Shiki, K. Ariga and K. Yamamoto:
trolyte, however, restricts the open-circuit voltage and J. Am. Chem. Soc., 123, 4414–4420 (2001).
the energy conversion efficiency at the interface. This [8] N. Satoh, J.-S. Cho, M. Higuchi and K. Yamamoto:
problem is resolved by TPA-DPAs, cast on the dye J. Photopol. Sci. Technol., 18, 55–58 (2005).
absorbing titanium oxide film, which assists the hole [9] M. Maciejewski: J. Macrom. Sci. Chem., 17A,
transfer (the reduction of the dye, oxidized by the pri- 689–703 (1982).
mary reaction) and suppress the back electron transfer [10] M.F. Ottaviani, F. Montalti, N.J. Turro and D.A.
from the titanium oxide (Fig. 19.4). Furthermore, the Tomalia: J. Phys. Chem. B, 101, 158–166 (1997).
accepter in this back electron transfer, I , complexes [11] L. Balogh, D.A. Tomalia: J. Am. Chem. Soc., 120,
3
with the imines at the branch structure of the den-
drimer at a ratio of 1:2, and then produces I working 7355–7356 (1998).
as an electron donor to the oxidized dye. [12] O. Varnavski, R.G. Ispasoiu, L. Balogh, D. Tomalia and
Because of the above-mentioned synergy effect, the T. Goodson III: J. Chem. Phys., 114, 1962–1965 (2001).
open-circuit voltage improves with the generational [13] R.W.J. Scott, O.M. Wilson and R.M. Crooks: J. Phys.
growth of the dendrimer. Moreover, the complexion with Chem. B, 109, 692–704 (2005).
497