Page 524 - Book Hosokawa Nanoparticle Technology Handbook
P. 524
APPLICATIONS 19 DENDRIMERS AND THEIR APPLICATION TO ORGANIC ELECTRONICS DEVICES
loss temperature is over 500ºC. Their rigid spherical leading to higher imine basicity in the inner layers [14].
structure forms a supramolecular assembly, the close The metal assembly reduces the electric resistance of
packing structure on the substrate by only cast [7, 8]. the dendrimer, which improves device performances.
Furthermore, the triphenylamine core is well known as
a hole transfer material and a electron donor.
3. Application to electronic devices
2. Metal assembling property of dendrimer The good solubility and easiness in preparing a
homogeneous film are also absolutely necessary for
Maciejewski proposed an idea to use the highly a material used in organic devices; dendrimers satisfy
branched molecules as a container holding the finer the requirement. Additionally, dendrimers isolate a
particles in the pores [9]. The host–guest chemistry chromophore by the dendritic shell.
using dendrimers has developed remarkably and estab- Yokoyama et al. applied the dendrimers, fixed rho-
lished an important position in supramolecular science. damine on the core, to the organic optical electronics
Tomalia et al. analyzed the metal assembly of Cu(II) like the optical amplification luminescence and solid
ions of a polyamide amine (PAMAM) dendrimer in laser [15]. The confinement of dyes within dendrimers
water, using ESR [10]. A Cu(II) ion combines with the suppresses the energy transfer between the dyes, espe-
four nitrogen atoms of the amine and amino groups in cially the self quenching in high-concentration solution
the dendrimer. Thus, the terminal amino groups of the or solid state [16]. The systems like this, including rho-
fourth generation dendrimer can hold maximum 15.5 damine, coumalin or DCM dyes, are also reported as
equivalent Cu(II) ions. The reduction of the included luminescent materials in high concentration [17, 18].
metals produces the metal clusters, held stably in the These methods are also applied to a light emitting
dendrimer [11]. The Au clusters, made by this method, layer in OLEDs. The blue light-emitting dye or poly-
show strong fluorescence. It is the first time as a metal mer in solid state is difficult, because of the red shift
cluster that an ultra fast attenuation of the fluorescence in luminescent wavelength and the self-quenching.
confirmed [12]. Additionally, Crooks et al. applied the Müllen and List et al. obtained the high efficient blue
included metal clusters to the catalysts [13]. OLEDs to synthesize the novel polyfluorenes,
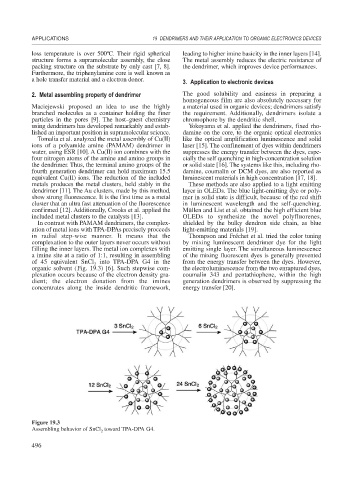
In contrast with PAMAM dendrimers, the complex- shielded by the bulky dendron side chain, as blue
ation of metal ions with TPA-DPAs precisely proceeds light-emitting materials [19].
in radial step-wise manner. It means that the Thompson and Fréchet et al. tried the color tuning
complexation to the outer layers never occurs without by mixing luminescent dendrimer dye for the light
filling the inner layers. The metal ion complexes with emitting single layer. The simultaneous luminescence
a imine site at a ratio of 1:1, resulting in assembling of the mixing fluorescent dyes is generally prevented
of 45 equivalent SnCl into TPA-DPA G4 in the from the energy transfer between the dyes. However,
2
organic solvent (Fig. 19.3) [6]. Such stepwise com- the electroluminescence from the two enraptured dyes,
plexation occurs because of the electron density gra- coumalin 343 and pentathiophene, within the high
dient; the electron donation from the imines generation dendrimers is observed by suppressing the
concentrates along the inside dendritic framework, energy transfer [20].
Figure 19.3
Assembling behavior of SnCl toward TPA-DPA G4.
2
496