Page 58 - Book Hosokawa Nanoparticle Technology Handbook
P. 58
1.9 SURFACE CHARACTERISTICS FUNDAMENTALS
Table 1.9.2
(a) Isoelectric point of various powders.
Material Isoelectric point
WO 3 2 1.8–2.5
0.5
SiO
Γ ( mol/m 2 ) water SiC 2 5.0–5.2
3–4
4.3
Au
Al(OH)
n-octane
SnO
2
6.7
-FeO(OH) 6.6
TiO 2 6.7
CeO 2 6.75
Cr O 3 7.0
2
-FeO(OH) 7.4
Zn(OH) 7.8
ln(p/p ) -Al O 3 2 7.4–8.6
0
2
Y O 3 9.0
2
-Fe O 3 9.04
2
(b) -Al O 3 9.1
2
ZnO 9.3
CuO 9.4
BeO 10.2
La O 3 10–11
10.4
2
(Γ) (mN/m) water Ni(OH) 2 2 12.4 0.3
ZrO
2
11.1
11.4
Co(OH)
n-octane
MgO
group to receive protons depends on acidity and
basicity, polarity and amount of charges differ from
one oxide to another. Table 1.9.2 shows isoelectric
point (pH at which interface charge is zero) of major
2
Γ ( mol/m ) oxides [10].
The features of the receptors of H and OH influ-
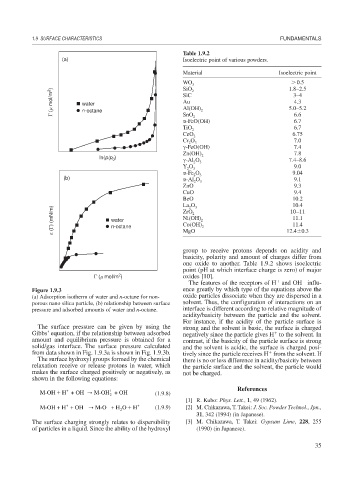
Figure 1.9.3 ence greatly by which type of the equations above the
(a) Adsorption isotherm of water and n-octane for non- oxide particles dissociate when they are dispersed in a
porous nano silica particle, (b) relationship between surface solvent. Thus, the configuration of interactions on an
pressure and adsorbed amounts of water and n-octane. interface is different according to relative magnitude of
acidity/basicity between the particle and the solvent.
For instance, if the acidity of the particle surface is
The surface pressure can be given by using the strong and the solvent is basic, the surface is charged
Gibbs’ equation, if the relationship between adsorbed negatively since the particle gives H to the solvent. In
amount and equilibrium pressure is obtained for a contrast, if the basicity of the particle surface is strong
solid/gas interface. The surface pressure calculated and the solvent is acidic, the surface is charged posi-
from data shown in Fig. 1.9.3a is shown in Fig. 1.9.3b. tively since the particle receives H from the solvent. If
The surface hydroxyl groups formed by the chemical there is no or less difference in acidity/basicity between
relaxation receive or release protons in water, which the particle surface and the solvent, the particle would
makes the surface charged positively or negatively, as not be charged.
shown in the following equations:
+
M-OH H + OH M-OH + OH (1.9.8) References
2
[1] R. Kubo: Phys. Lett., 1, 49 (1962).
+
M-OH + H + OH M-O + H O H + (1.9.9) [2] M. Chikazawa, T. Takei: J. Soc. Powder Technol., Jpn.,
2
31, 342 (1994) (in Japanese).
The surface charging strongly relates to dispersibility [3] M. Chikazawa, T. Takei: Gypsum Lime, 228, 255
of particles in a liquid. Since the ability of the hydroxyl (1990) (in Japanese).
35