Page 591 - Book Hosokawa Nanoparticle Technology Handbook
P. 591
34 DEVELOPMENT OF PHOTOCATALYST APPLICATIONS
TiO was evaluated with a gas detector under UV decreased formaldehyde to 7 ppm after only 24 h
2
2
light (1 mW/cm ) or in the dark. The original alumi- under UV light. Then, the gas was injected again; the
nosilicate decreased formaldehyde concentration to gas concentration also decreased down to 10 ppm
around 14 ppm under both conditions as shown in after 24 h. The results suggest that the TiO complex
2
Fig. 34.5. The difference between without and with showed photocatalysis.
UV light was not observed. Accordingly, this To clarify the photocatalysis of TiO complex, the
2
decrease seems to be due to absorption. However, as carbondioxide (CO ) that was generated by decom-
2
shown in Fig. 34.6 the TiO complex drastically posing formaldehyde was determined quantitatively
2
by FT-IR analysis as shown in Fig. 34.7 [6]. The ini-
tial formaldehyde of 180 ppm was adsorbed down to
almost 45 ppm on the TiO complex without UV light,
2
because CO was not generated. After the irradiation
2
of UV light began, the concentration of CO increased
2
with a decrease in that of formaldehyde.
In order to investigate the difference of the photo-
catalytic efficiency between the TiO –aluminosilicate
2
complex and the powder (TiO mixture) prepared
2
from TiO and aluminosilicate by dry blending, the
2
decomposition of formaldehyde was examined as a
function of the TiO quantity. When the initial
2
concentration of formaldehyde was fixed at 20 ppm,
the formaldehyde concentrations after 2 h under UV
light are shown in Fig. 34.8. The TiO complexes with
2
1- and 5-wt.% TiO efficiently decomposed formalde-
2
hyde compared to the TiO mixtures. In particular, the
2
TiO 2 complex with 5 wt.% TiO 2 decomposed
formaldehyde down to 4 ppm, though the TiO mix-
2
ture hardly decomposed it. It seems that the reason
why TiO complex has high efficiency is because of
2
good dispersibility of the TiO nanoparticles on the
40 nm 2
surface of aluminosilicate. However, when the
amount of TiO was 10 wt.%, the formaldehyde con-
2
centration of both powders was about 5ppm. The rea-
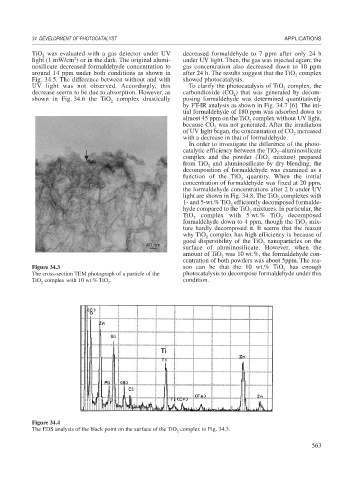
Figure 34.3 son can be that the 10 wt.% TiO has enough
2
The cross-section TEM photograph of a particle of the photocatalysis to decompose formaldehyde under this
TiO complex with 10 wt.% TiO . condition.
2
2
Ti
Figure 34.4
The EDS analysis of the black point on the surface of the TiO complex in Fig. 34.3.
2
563