Page 592 - Book Hosokawa Nanoparticle Technology Handbook
P. 592
APPLICATIONS 34 DEVELOPMENT OF PHOTOCATALYST
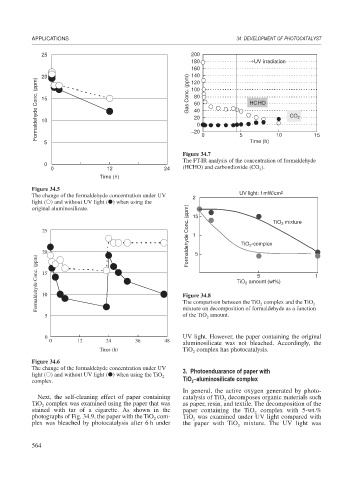
25 200
180 → UV irradiation
160
20 140
Formaldehyde Conc. (ppm) 15 Gas Conc. (ppm) 80 0 HCHO CO 2
120
100
60
40
20
10
5 −20 0 5 Time (h) 10 15
Figure 34.7
The FT-IR analysis of the concentration of formaldehyde
0
0 12 24 (HCHO) and carbondioxide (CO ).
2
Time (h)
Figure 34.5
The change of the formaldehyde concentration under UV 20 UV light: 1 mW/cm 2
light ( ) and without UV light ( ) when using the
Formaldehyde Conc. (ppm) 10
original aluminosilicate. 15
25 TiO 2 mixture
20 5 TiO 2 -complex
Formaldehyde Conc. (ppm) 15 Figure 34.8 TiO 2 amount (wt%) 10
0
0
5
10
2
mixture on decomposition of formaldehyde as a function
of the TiO amount.
5 The comparison between the TiO complex and the TiO 2
2
0 UV light. However, the paper containing the original
0 12 24 36 48
aluminosilicate was not bleached. Accordingly, the
Time (h) TiO complex has photocatalysis.
2
Figure 34.6
The change of the formaldehyde concentration under UV 3. Photoenduarance of paper with
light ( ) and without UV light ( ) when using the TiO
2
complex. TiO –aluminosilicate complex
2
In general, the active oxygen generated by photo-
Next, the self-cleaning effect of paper containing catalysis of TiO decomposes organic materials such
2
TiO complex was examined using the paper that was as paper, resin, and textile. The decomposition of the
2
stained with tar of a cigarette. As shown in the paper containing the TiO complex with 5-wt.%
2
photographs of Fig. 34.9, the paper with the TiO com- TiO was examined under UV light compared with
2
2
plex was bleached by photocatalysis after 6 h under the paper with TiO mixture. The UV light was
2
564