Page 590 - Book Hosokawa Nanoparticle Technology Handbook
P. 590
APPLICATIONS 34 DEVELOPMENT OF PHOTOCATALYST
APPLICATION 34
34 DEVELOPMENT OF PHOTOCATALYST INSERTED INTO SURFACE OF POROUS
ALUMINOSILICATE
Anatase-type TiO with nanosize particles is well observed. The pore-size distribution was determined
2
known as a photocatalyst [1]. The TiO absorbs to be from 4 to 100 nm by using N adsorption–des-
2
2
ultraviolet light (UV) of 390 nm or less and generates orption isotherms by the BET (Brunauer Emmitt and
active oxygen such as hydroxyl radicals and the super Teller) method (Fig. 34.2). The surface area was 160
2
anion oxide by the oxidation of water and oxygen in m /g. Moreover, this aluminosilicate has the
air. The active oxygen with a strong oxidizing activity deodorizing effects of some gases such as ammonia,
decomposes organic compounds, and demonstrates hydrogen sulfite, and acetic acid, because its compo-
self-cleaning, deodorizing, and an anti-bacterial sition is 8.6-SiO –Al O –4.8-ZnO and has acid and
2
3
2
effect. However, the photocatalyst has some disadvan- alkaline functional groups on the surface.
tages. For example, the active oxygen generated by Using colloidal TiO (STS-1 from Ishihara Sangyo
2
TiO generally decomposes organic materials such as Co., Ltd. Japan) as a photocatalyst, the TiO com-
2
2
paper, resin, and textile [2,3]. The TiO cannot be plexes containing 1, 5 and 10 wt.% of TiO were pre-
2
2
processed into the organic materials because of a pared from the TiO and the porous aluminosilicate by
2
strong oxidizing activity. Next, its efficiency is not using a cationic surfactant. As the cross-section TEM
enough for each practical condition because of a lack (Transmission Electron Microscope) photograph of a
of adsorption capacity. On the other hand, it is diffi- particle of the TiO complex is shown in Fig. 34.3,
2
cult for a consumer to recognize its performance. several black points with about 10 nm were observed
Accordingly, to solve these problems, the photocata- near the surface of aluminosilicate. The Ti element
lyst inserted into a porous aluminosilicate, which has was detected only on the black points by EDS (Energy
anti-bacterial and deodorizing effects, was developed. Dispersive X ray Spectrometer) analysis in Fig. 34.4.
Characteristics of this TiO –aluminosilicate complex These results suggest that the TiO nanoparticles of
2
2
will be introduced in this chapter [4, 5]. the Ti complex were inserted into the pore near the
surface of aluminosilicate.
1. Structure of TiO –aluminosilicate complex
2
2. Photocatalysis of TiO –aluminosilicate complex
2
The mean particle size of a porous aluminosilicate
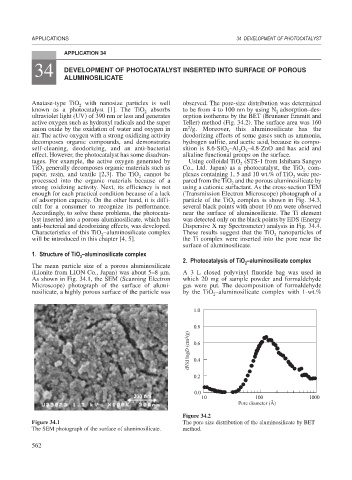
(Lionite from LION Co., Japan) was about 5–8 m. A 3 L closed polyvinyl fluoride bag was used in
As shown in Fig. 34.1, the SEM (Scanning Electron which 20 mg of sample powder and formaldehyde
Microscope) photograph of the surface of alumi- gas were put. The decomposition of formaldehyde
nosilicate, a highly porous surface of the particle was by the TiO –aluminosilicate complex with 1-wt.%
2
1.0
0.8
dV/d logD (cm 3 /g) 0.6
0.4
0.2
0.0
300 nm 10 100 1000
Pore diameter (Å)
Figure 34.2
Figure 34.1 The pore size distribution of the aluminosilicate by BET
The SEM photograph of the surface of aluminosilicate. method.
562