Page 409 - Organic Electronics in Sensors and Biotechnology
P. 409
386 Chapter Ten
functionalized multiwalled carbon nanotubes (MWCNT-c) by using
the electrophoretic technique. The results of the CV and EIS
(electrochemical impedance spectroscopy) studies indicate enhanced
electrochemical and charge transfer behavior of the composite as
compared to pure polyaniline. This enhanced electrochemical response
in electrophoretically deposited ES/MWCNT-c composite has been
utilized to improve characteristics of biosensors. The application of
ES/MWCNT-c/ITO electrode to biosensor for cholesterol indicates
short response time (10 s) and high sensitivity (6800 nA/mM). This
enhanced sensitivity is attributed to the incorporation of the MWCNT-c
in the matrix and to the intimate association obtained between these
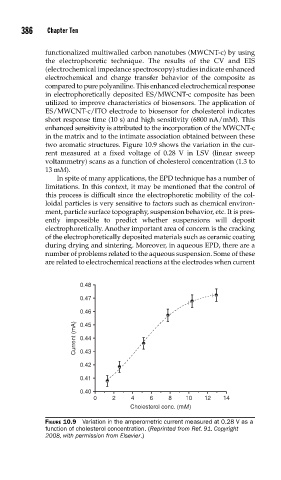
two aromatic structures. Figure 10.9 shows the variation in the cur-
rent measured at a fixed voltage of 0.28 V in LSV (linear sweep
voltammetry) scans as a function of cholesterol concentration (1.3 to
13 mM).
In spite of many applications, the EPD technique has a number of
limitations. In this context, it may be mentioned that the control of
this process is difficult since the electrophoretic mobility of the col-
loidal particles is very sensitive to factors such as chemical environ-
ment, particle surface topography, suspension behavior, etc. It is pres-
ently impossible to predict whether suspensions will deposit
electrophoretically. Another important area of concern is the cracking
of the electrophoretically deposited materials such as ceramic coating
during drying and sintering. Moreover, in aqueous EPD, there are a
number of problems related to the aqueous suspension. Some of these
are related to electrochemical reactions at the electrodes when current
0.48
0.47
0.46
Current (mA) 0.45
0.44
0.43
0.42
0.41
0.40
0 2 4 6 8 10 12 14
Cholesterol conc. (mM)
FIGURE 10.9 Variation in the amperometric current measured at 0.28 V as a
function of cholesterol concentration. (Reprinted from Ref. 91. Copyright
2008, with permission from Elsevier.)