Page 126 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 126
4.3 Eigenfunctions 117
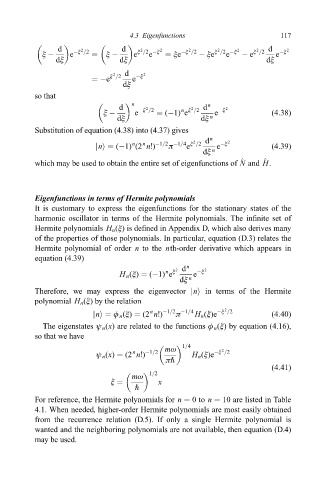
d 2 d 2 2 2 2 2 2 d 2
e
e
î ÿ e ÿî =2 î ÿ e î =2 ÿî îe ÿî =2 ÿ îe î =2 ÿî ÿ e î =2 e ÿî
dî dî dî
2
ÿe î =2 d e ÿî 2
dî
so that
n n
d ÿî =2 n î =2 d ÿî 2
2
2
î ÿ e (ÿ1) e e (4:38)
dî dî n
Substitution of equation (4.38) into (4.37) gives
2
n
ð
n
jni (ÿ1) (2 n!) ÿ1=2 ÿ1=4 î =2 d n e ÿî 2 (4:39)
e
dî n
^
^
which may be used to obtain the entire set of eigenfunctions of N and H.
Eigenfunctions in terms of Hermite polynomials
It is customary to express the eigenfunctions for the stationary states of the
harmonic oscillator in terms of the Hermite polynomials. The in®nite set of
Hermite polynomials H n (î) is de®ned in Appendix D, which also derives many
of the properties of those polynomials. In particular, equation (D.3) relates the
Hermite polynomial of order n to the nth-order derivative which appears in
equation (4.39)
n î
H n (î) (ÿ1) e 2 d n e ÿî 2
dî n
Therefore, we may express the eigenvector jni in terms of the Hermite
polynomial H n (î) by the relation
2
n
ð
jni ö n (î) (2 n!) ÿ1=2 ÿ1=4 H n (î)e ÿî =2 (4:40)
The eigenstates ø n (x) are related to the functions ö n (î) by equation (4.16),
so that we have
1=4
mù 2
n
ø n (x) (2 n!) ÿ1=2 H n (î)e ÿî =2
ð"
(4:41)
1=2
mù
î x
"
For reference, the Hermite polynomials for n 0to n 10 are listed in Table
4.1. When needed, higher-order Hermite polynomials are most easily obtained
from the recurrence relation (D.5). If only a single Hermite polynomial is
wanted and the neighboring polynomials are not available, then equation (D.4)
may be used.