Page 129 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 129
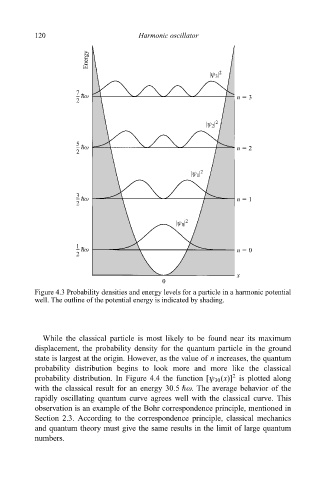
120 Harmonic oscillator
Energy
|ψ | 2
3
7
hω n 5 3
2
|ψ | 2
2
5
hω n 5 2
2
|ψ | 2
1
3
hω n 5 1
2
|ψ 0 | 2
1
hω n 5 0
2
x
0
Figure 4.3 Probability densities and energy levels for a particle in a harmonic potential
well. The outline of the potential energy is indicated by shading.
While the classical particle is most likely to be found near its maximum
displacement, the probability density for the quantum particle in the ground
state is largest at the origin. However, as the value of n increases, the quantum
probability distribution begins to look more and more like the classical
2
probability distribution. In Figure 4.4 the function [ø 30 (x)] is plotted along
with the classical result for an energy 30:5 "ù. The average behavior of the
rapidly oscillating quantum curve agrees well with the classical curve. This
observation is an example of the Bohr correspondence principle, mentioned in
Section 2.3. According to the correspondence principle, classical mechanics
and quantum theory must give the same results in the limit of large quantum
numbers.