Page 147 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 147
138 Angular momentum
5.3 Application to orbital angular momentum
We now apply the results of the quantum-mechanical treatment of generalized
angular momentum to the case of orbital angular momentum. The orbital
^
angular momentum operator L, de®ned in Section 5.1, is identi®ed with the
^
^
^
^ 2 ^
operator J of Section 5.2. Likewise, the operators L , L x , L y , and L z are
^ 2 ^
^
^
identi®ed with J , J x , J y , and J z , respectively. The parameter j of Section 5.2
is denoted by l when applied to orbital angular momentum. The simultaneous
^
^ 2
eigenfunctions of L and L z are denoted by jlmi, so that we have
^ 2 2
L jlmi l(l 1)" jlmi (5:28a)
^ m ÿl, ÿl 1, ... , l ÿ 1, l (5:28b)
L z jlmi m"jlmi,
Our next objective is to ®nd the analytical forms for these simultaneous
eigenfunctions. For that purpose, it is more convenient to express the operators
^ ^ ^ ^ 2
L x , L y , L z , and L in spherical polar coordinates r, è, j rather than in cartesian
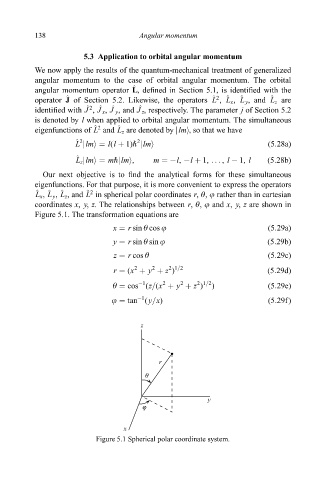
coordinates x, y, z. The relationships between r, è, j and x, y, z are shown in
Figure 5.1. The transformation equations are
x r sin è cos j (5:29a)
y r sin è sin j (5:29b)
z r cos è (5:29c)
2 1=2
2
2
r (x y z ) (5:29d)
2
2 1=2
2
ÿ1
è cos (z=(x y z ) ) (5:29e)
ÿ1
j tan (y=x) (5:29f)
z
r
θ
y
j
x
Figure 5.1 Spherical polar coordinate system.