Page 148 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 148
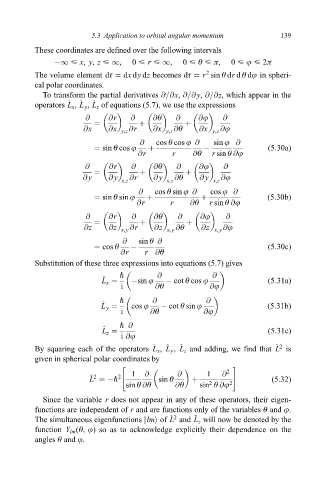
5.3 Application to orbital angular momentum 139
These coordinates are de®ned over the following intervals
ÿ1 < x, y, z < 1, 0 < r < 1, 0 < è < ð, 0 < j < 2ð
2
The volume element dô dx dy dz becomes dô r sin è dr d è dj in spheri-
cal polar coordinates.
To transform the partial derivatives @=@x, @=@ y, @=@z, which appear in the
^
^
^
operators L x , L y , L z of equations (5.7), we use the expressions
@ @r @ @è @ @j @
@x @x y,z @r @x y,z @è @x y,z @j
@ cos è cos j @ sin j @
sin è cos j ÿ (5:30a)
@r r @è r sin è @j
@ @r @ @è @ @j @
@ y @ y @r @ y @è @ y @j
x,z x,z x,z
@ cos è sin j @ cos j @
sin è sin j (5:30b)
@r r @è r sin è @j
@ @r @ @è @ @j @
@z @z x, y @r @z x, y @è @z x, y @j
@ sin è @
cos è ÿ (5:30c)
@r r @è
Substitution of these three expressions into equations (5.7) gives
" @ @
^ ÿsin j ÿ cot è cos j
L x (5:31a)
i @è @j
" @ @
^ cos j ÿ cot è sin j
L y (5:31b)
i @è @j
" @
^ (5:31c)
L z
i @j
^
^
^
^ 2
By squaring each of the operators L x , L y , L z and adding, we ®nd that L is
given in spherical polar coordinates by
" #
2
^ 2 2 1 @ sin è @ 1 @ (5:32)
L ÿ"
2
sin è @è @è sin è @j 2
Since the variable r does not appear in any of these operators, their eigen-
functions are independent of r and are functions only of the variables è and j.
^
^ 2
The simultaneous eigenfunctions jlmi of L and L z will now be denoted by the
function Y lm (è, j) so as to acknowledge explicitly their dependence on the
angles è and j.