Page 70 - PVT Property Correlations
P. 70
48 PVT Property Correlations
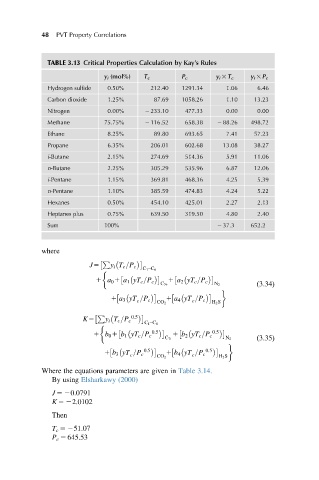
TABLE 3.13 Critical Properties Calculation by Kay’s Rules
y i (mol%) T c P c y i 3 T c y i 3 P c
Hydrogen sulfide 0.50% 212.40 1291.34 1.06 6.46
Carbon dioxide 1.25% 87.69 1058.26 1.10 13.23
Nitrogen 0.00% 2 233.10 477.33 0.00 0.00
Methane 75.75% 2 116.52 658.38 2 88.26 498.72
Ethane 8.25% 89.80 693.65 7.41 57.23
Propane 6.35% 206.01 602.68 13.08 38.27
i-Butane 2.15% 274.69 514.36 5.91 11.06
n-Butane 2.25% 305.29 535.96 6.87 12.06
i-Pentane 1.15% 369.81 468.36 4.25 5.39
n-Pentane 1.10% 385.59 474.83 4.24 5.22
Hexanes 0.50% 454.10 425.01 2.27 2.13
Heptanes plus 0.75% 639.50 319.50 4.80 2.40
Sum 100% 2 37.3 652.2
where
J5 P
y i T c =P c
C 1 2C 6
1 a 0 1 a 1 yT c =P c 1 a 2 yT c =P c
C 71 N 2 ð3:34Þ
1 a 3 yT =P c 1 a 4 yT =P c H 2 S
c
c
CO 2
K5 P 0:5
y i T c =P c
C 1 2C 6
0:5 0:5
1 b 0 1 b 1 yT c =P c 1 b 2 yT c =P c
C 71 N 2 ð3:35Þ
0:5 0:5
1 b 3 yT =P c 1 b 4 yT =P c H 2 S
c
c
CO 2
Where the equations parameters are given in Table 3.14.
By using Elsharkawy (2000)
J 520.0791
K 522.0102
Then
T c 5251.07
P c 5 645.53