Page 75 - PVT Property Correlations
P. 75
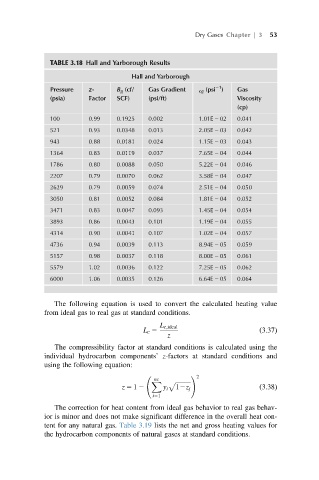
Dry Gases Chapter | 3 53
TABLE 3.18 Hall and Yarborough Results
Hall and Yarborough
21
Pressure z- B g (cf/ Gas Gradient cg (psi ) Gas
(psia) Factor SCF) (psi/ft) Viscosity
(cp)
100 0.99 0.1925 0.002 1.01E 2 02 0.041
521 0.93 0.0348 0.013 2.05E 2 03 0.042
943 0.88 0.0181 0.024 1.15E 2 03 0.043
1364 0.83 0.0119 0.037 7.65E 2 04 0.044
1786 0.80 0.0088 0.050 5.22E 2 04 0.046
2207 0.79 0.0070 0.062 3.58E 2 04 0.047
2629 0.79 0.0059 0.074 2.51E 2 04 0.050
3050 0.81 0.0052 0.084 1.81E 2 04 0.052
3471 0.83 0.0047 0.093 1.45E 2 04 0.054
3893 0.86 0.0043 0.101 1.19E 2 04 0.055
4314 0.90 0.0041 0.107 1.02E 2 04 0.057
4736 0.94 0.0039 0.113 8.94E 2 05 0.059
5157 0.98 0.0037 0.118 8.00E 2 05 0.061
5579 1.02 0.0036 0.122 7.25E 2 05 0.062
6000 1.06 0.0035 0.126 6.64E 2 05 0.064
The following equation is used to convert the calculated heating value
from ideal gas to real gas at standard conditions.
L c;ideal
L c 5 ð3:37Þ
z
The compressibility factor at standard conditions is calculated using the
individual hydrocarbon components’ z-factors at standard conditions and
using the following equation:
! 2
nc
ffiffiffiffiffiffiffiffiffiffi
X p
z 5 1 2 y i 12z j ð3:38Þ
i51
The correction for heat content from ideal gas behavior to real gas behav-
ior is minor and does not make significant difference in the overall heat con-
tent for any natural gas. Table 3.19 lists the net and gross heating values for
the hydrocarbon components of natural gases at standard conditions.