Page 74 - PVT Property Correlations
P. 74
52 PVT Property Correlations
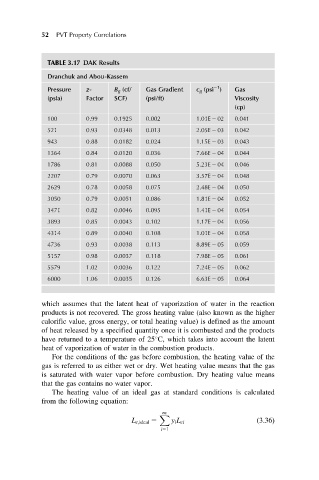
TABLE 3.17 DAK Results
Dranchuk and Abou-Kassem
21
Pressure z- B g (cf/ Gas Gradient c g (psi ) Gas
(psia) Factor SCF) (psi/ft) Viscosity
(cp)
100 0.99 0.1925 0.002 1.01E 2 02 0.041
521 0.93 0.0348 0.013 2.05E 2 03 0.042
943 0.88 0.0182 0.024 1.15E 2 03 0.043
1364 0.84 0.0120 0.036 7.66E 2 04 0.044
1786 0.81 0.0088 0.050 5.23E 2 04 0.046
2207 0.79 0.0070 0.063 3.57E 2 04 0.048
2629 0.78 0.0058 0.075 2.48E 2 04 0.050
3050 0.79 0.0051 0.086 1.81E 2 04 0.052
3471 0.82 0.0046 0.095 1.41E 2 04 0.054
3893 0.85 0.0043 0.102 1.17E 2 04 0.056
4314 0.89 0.0040 0.108 1.01E 2 04 0.058
4736 0.93 0.0038 0.113 8.89E 2 05 0.059
5157 0.98 0.0037 0.118 7.98E 2 05 0.061
5579 1.02 0.0036 0.122 7.24E 2 05 0.062
6000 1.06 0.0035 0.126 6.63E 2 05 0.064
which assumes that the latent heat of vaporization of water in the reaction
products is not recovered. The gross heating value (also known as the higher
calorific value, gross energy, or total heating value) is defined as the amount
of heat released by a specified quantity once it is combusted and the products
have returned to a temperature of 25 C, which takes into account the latent
heat of vaporization of water in the combustion products.
For the conditions of the gas before combustion, the heating value of the
gas is referred to as either wet or dry. Wet heating value means that the gas
is saturated with water vapor before combustion. Dry heating value means
that the gas contains no water vapor.
The heating value of an ideal gas at standard conditions is calculated
from the following equation:
nc
X
L c;ideal 5 y i L ci ð3:36Þ
i51