Page 110 - Petroleum and Gas Field Processing
P. 110
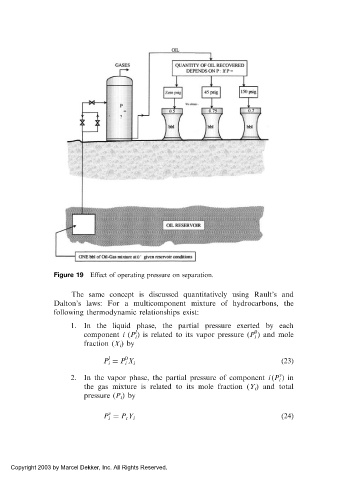
Figure 19 Effect of operating pressure on separation.
The same concept is discussed quantitatively using Rault’s and
Dalton’s laws: For a multicomponent mixture of hydrocarbons, the
following thermodynamic relationships exist:
1. In the liquid phase, the partial pressure exerted by each
l 0
component i ðP i Þ is related to its vapor pressure ðP i Þ and mole
fraction (X i )by
l 0
P i ¼ P i X i ð23Þ
v
2. In the vapor phase, the partial pressure of component i ðP i Þ in
the gas mixture is related to its mole fraction (Y i ) and total
pressure (P t )by
v
P i ¼ P t Y i ð24Þ
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.