Page 160 - Photoreactive Organic Thin Films
P. 160
4. PHOTO1SOHERIZAT1ON AND PHOTO-ORIENTATION OF AZO DYE IN FILMS OF POLYMER 139
(4.2)
where AV* is the activation volume, that is, the minimum volume needed for
the reaction to proceed, P is the pressure, T is the absolute temperature, R is
the universal gas constant, and k 0 is the reaction rate that corresponds to the
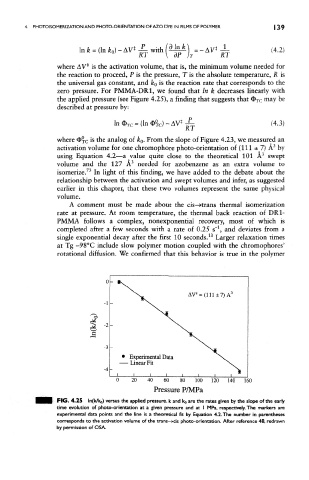
zero pressure. For PMMA-DR1, we found that In k decreases linearly with
the applied pressure (see Figure 4.25), a finding that suggests that O TC may be
described at pressure by:
(4.3)
where <E>xc * s tne analog of k Q. From the slope of Figure 4.23, we measured an
3
activation volume for one chromophore photo-orientation of (111 ± 7) A by
3
using Equation 4.2—a value quite close to the theoretical 101 A swept
3
volume and the 127 A needed for azobenzene as an extra volume to
73
isomerize. In light of this finding, we have added to the debate about the
relationship between the activation and swept volumes and infer, as suggested
earlier in this chapter, that these two volumes represent the same physical
volume.
A comment must be made about the cis-»trans thermal isomerization
rate at pressure. At room temperature, the thermal back reaction of DR1-
PMMA follows a complex, nonexponential recovery, most of which is
1
completed after a few seconds with a rate of 0.25 s" , and deviates from a
13
single exponential decay after the first 10 seconds. Larger relaxation times
at Tg ~98°C include slow polymer motion coupled with the chromophores'
rotational diffusion. We confirmed that this behavior is true in the polymer
AV* = (111±7)A 3
_2
-3
Experimental Data
• Linear Fit
-4
20 40 60 80 100 120 140 160
Pressure P/MPa
FIG. 4.25 ln(k/ko) versus the applied pressure, k and ko are the rates given by the slope of the early
time evolution of photo-orientation at a given pressure and at I MPa, respectively. The markers are
experimental data points and the line is a theoretical fit by Equation 4.2. The number in parentheses
corresponds to the activation volume of the trans-»cis photo-orientation. After reference 48, redrawn
by permission of OSA.