Page 155 - Photoreactive Organic Thin Films
P. 155
134 ZOUHEIR SEKKAT AND WOLFGANG KNOLL
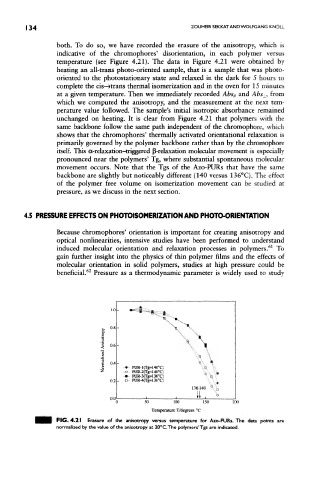
both. To do so, we have recorded the erasure of the anisotropy, which is
indicative of the chromophores' disorientation, in each polymer versus
temperature (see Figure 4.21). The data in Figure 4.21 were obtained by
heating an all-trans photo-oriented sample, that is a sample that was photo-
oriented to the photostationary state and relaxed in the dark for 5 hours to
complete the cis—»trans thermal isomerization and in the oven for 15 minutes
at a given temperature. Then we immediately recorded Abs// and Abs±, from
which we computed the anisotropy, and the measurement at the next tem-
perature value followed. The sample's initial isotropic absorbance remained
unchanged on heating. It is clear from Figure 4.21 that polymers with the
same backbone follow the same path independent of the chromophore, which
shows that the chromophores' thermally activated orientational relaxation is
primarily governed by the polymer backbone rather than by the chromophore
itself. This a-relaxation-triggered {^-relaxation molecular movement is especially
pronounced near the polymers' Tg, where substantial spontaneous molecular
movement occurs. Note that the Tgs of the Azo-PURs that have the same
backbone are slightly but noticeably different (140 versus 136°C). The effect
of the polymer free volume on isomerization movement can be studied at
pressure, as we discuss in the next section.
4.5 PRESSURE EFFECTS ON PHOTOISOMERIZATION AND PHOTO-ORIENTATION
Because chromophores' orientation is important for creating anisotropy and
optical nonlinearities, intensive studies have been performed to understand
61
induced molecular orientation and relaxation processes in polymers. To
gain further insight into the physics of thin polymer films and the effects of
molecular orientation in solid polymers, studies at high pressure could be
62
beneficial. Pressure as a thermodynamic parameter is widely used to study
1.0
0.8
• PUR-l(Tg=140°C)
o PUR-2(Tg=140°C)
-•-• PUR-3{Tg=136°C)
0.2 -D- PUR-4(Tg=136°C)
136140
II ,
0.0
50 100 150 200
Temperature I/degrees °C
FIG. 4.21 Erasure of the anisotropy versus temperature for Azo-PURs. The data points are
normalized by the value of the anisotropy at 20°C.The polymers' Tgs are indicated.