Page 201 - Photoreactive Organic Thin Films
P. 201
HENN1NG MENZEL
appropriate chromophore, however; the structure of the film-forming matrix
must also be tailored to meet the requirements to keep the chromophores
photoactive. Moreover, it is possible to design the matrix in a way that boosts
the photoindueed changes caused by the photoreaction and enhances the
system's response. Photoisomerization, a very common mechanism used In
photoactive films, is a process requiring some free volume and is, therefore,
strongly influenced by the matrix. But reciprocally, it also affects the matrix
in its structure and order.
Langmulr-Blodgett-Kuhn (LBK) films are prepared by a dipping. process
in which naonolayers floating at the air/water interface are deposited on a
solid substrate. LBK films are highly ordered supramolecular assemblies. By
selection of the arnphiphilic molecules and adjustment of the transfer condi-
tions, the LBK technique offers extensive structural control and opens a way
to optimize matrices for photoactive dyes.
6.2 OTHER DYES USED IN PHOTOACTIVE LBK FILMS
Several photoactive dyes have been incorporated into LBK films. Among
these, the class of photoisomerizing dyes, namely azobenzene derivatives, are
particularly versatile and have been investigated in great detail.
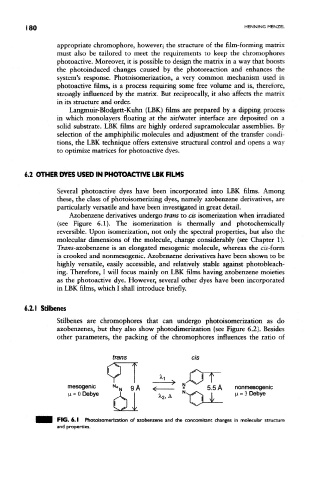
Azobenzene derivatives undergo trans to cis isomerization when irradiated
(see Figure 6.1). The isomerization is thermally and photochemkally
reversible. Upon isomerization, not only the spectral properties, but also the
molecular dimensions of the molecule, change considerably (see Chapter 1).
Traws-azobenzene is an elongated mesogenic molecule, whereas the ds-form
is crooked and nonmesogenic. Azobenzene derivatives have been shown to be
highly versatile, easily accessible, and relatively stable against photobleach-
ing. Therefore, I will focus mainly on LBK films having azobenzene moieties
as the photoactive dye. However, several other dyes have been incorporated
in LBK films, which I shall introduce briefly.
6.2.1 Stilbenes
Stilbenes are chromophores that can undergo photoisomerization as do
azobenzenes, but they also show photodimerization (see Figure 6.2). Besides
other parameters, the packing of the chromophores influences the ratio of
trans cis
mesogenic N« N g^ ^ jj 5,5 A nonmesogenic
(j. = 0 Debye
FIG. 6.1 Photoisomerization of azobenzene and the concomitant changes in molecular structure
and properties.