Page 205 - Photoreactive Organic Thin Films
P. 205
HENNING MENZEL
such as in densely packed LBK films, they are not fulfilled. The optical
properties of the chromophore depend on the dense packing in the LBK film
(see Section 6.3.2} and are changed when increasing amounts of ds-isotner
alter the packing of the chromophores, In this case, all spectroscopic methods
for determining the dsltrans ratio will fail. Besides spectroscopy, electro-
chemical methods can be employed to determine the dsltrans ratio in LBK
films. The a's-isomer can be reduced at significantly greater anodic potential
than the trans-isomer, this gives rise to a corresponding peak in the cyclic
voltammetry experiment, and the amount of c/s-isomer then can be calculated
from either the cathodic or the anodic charge. The total number of chro-
mophores can be calculated from the transfer conditions in the LBK experi-
21
ment. It is even possible to use this method for actinometry. It was by
employing this method that it was found that only 19% of chromophore 7
can be isomerized in LBK films. 18
6.3.2 Aggregation
As mentioned previously, the interactions of the chromophores with extended
Ti-systems in densely packed solids like LBK films significantly influence the
optical properties. In particular, the aggregation causes a significant peak
shift, the direction and extent of which depends on the number of aggregated
chromophores and their distance from each other in the aggregate.
Furthermore, a particular influence on the extent and direction of the peak
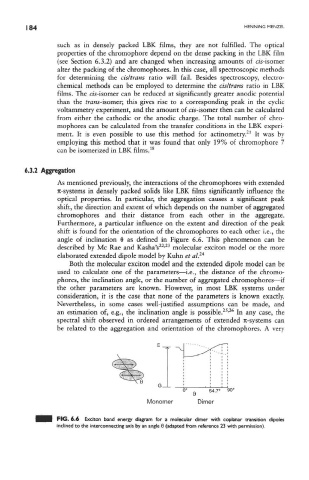
shift is found for the orientation of the chromophores to each other i.e., the
angle of inclination 6 as defined in Figure 6.6. This phenomenon can be
22 23
described by Me Rae and Kasha's ' molecular exciton model or the more
24
elaborated extended dipole model by Kuhn et al.
Both the molecular exciton model and the extended dipole model can be
used to calculate one of the parameters — i.e., the distance of the chromo-
phores, the inclination angle, or the number of aggregated chromophores — if
the other parameters are known. However, in most LBK systems under
consideration, it is the case that none of the parameters is known exactly.
Nevertheless, in some cases well- justified assumptions can be made, and
25 26
an estimation of, e.g., the inclination angle is possible. ' In any case, the
spectral shift observed in ordered arrangements of extended 7C-systems can
be related to the aggregation and orientation of the chromophores. A very
FIG. 6.6 Exciton band energy diagram for a molecular dimer with coplanar transition dipoles
inclined to the interconnecting axis by an angle 0 (adapted from reference 23 with permission).