Page 207 - Photoreactive Organic Thin Films
P. 207
186 HENNING MENZEL
60
335
50 -
330
40 -
£
I 3°
CD 325
CL
« 20 H
10 - 320
o -
0.00 0.25 0.50 0.75 1.00 1.25 1.50 1.75
2
Area per Amphiphile [nm ]
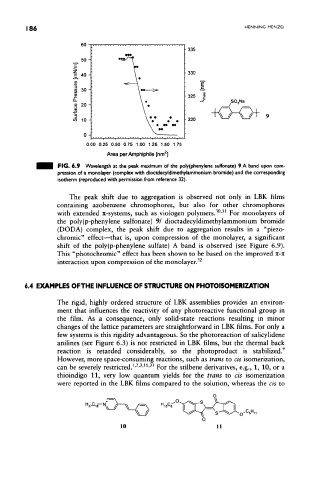
FIG. 6.9 Wavelength at the peak maximum of the poly(phenylene sulfonate) 9 A band upon com-
pression of a monolayer (complex with dioctdecyldimethylammonium bromide) and the corresponding
isotherm (reproduced with permission from reference 32).
The peak shift due to aggregation is observed not only in LBK films
containing azobenzene chromophores, but also for other chromophores
30 31
with extended Tt-systems, such as viologen polymers. ' For monolayers of
the poly(p-phenylene sulfonate) 91 dioctadecyldimethylammonium bromide
(DODA) complex, the peak shift due to aggregation results in a "piezo-
chromic" effect—that is, upon compression of the monolayer, a significant
shift of the poly(p-phenylene sulfate) A band is observed (see Figure 6.9).
This "photochromic" effect has been shown to be based on the improved n-n
interaction upon compression of the monolayer. 32
6.4 EXAMPLES OFTHE INFLUENCE OF STRUCTURE ON PHOTOISOMERIZATION
The rigid, highly ordered structure of LBK assemblies provides an environ-
ment that influences the reactivity of any photoreactive functional group in
the film. As a consequence, only solid-state reactions resulting in minor
changes of the lattice parameters are straightforward in LBK films. For only a
few systems is this rigidity advantageous. So the photoreaction of salicylidene
anilines (see Figure 6.3) is not restricted in LBK films, but the thermal back
9
reaction is retarded considerably, so the photoproduct is stabilized.
However, more space-consuming reactions, such as trans to cis isomerization,
1 2 3 11 33
can be severely restricted. ' ' ' ' For the stilbene derivatives, e.g., 1, 10, or a
thioindigo 11, very low quantum yields for the trans to cis isomerization
were reported in the LBK films compared to the solution, whereas the cis to
10