Page 212 - Photoreactive Organic Thin Films
P. 212
6 PHOTOISOMER1ZATION SN LANGMU1R-BLODGETT-KUHN STRUCTURES J 9 J
Tethering azobenzene moieties to a crown-ether as polar head group (18)
gives an amphiphile that forms monolayers at the air/water interface. The
large crown-ether functionality provides enough free volume for azobenzene
trans to cis isomerization. The photochemical conversion in this azobenzene
53
nionolayer reached 76%, showing a great increase compared with that in a
simple long-chain fatty acid azobenzene (7) LBK film (30% determined by
18
spectroscopy, 19% determined by electrochemical methods. The percentage
is only a little smaller than the value obtained in solution (86%).
In addition to crown-ethers, calix[4]resorcinarenes 19 have also been
used as head groups to obtain azobenzene amphiphiles with sufficient photo-
isomerization in LBK films. For this purpose, azobenzene moieties have been
tethered to the lower rim of the crown conformer of the calixarene.
O-octacarboxymethoxylated calix[4]resorcinarenes 19 (X = CH 2-COOH)
display efficient trans to cis photoisomerizability in densely packed mono-
layers on a water surface, in LBK films, and in surface-adsorbed monolayers,
whereas the noncarboxymetylated 19 (X = H) derivative gives films that are
too densely packed. However, the aggregation is already suppressed efficiently
54 55
compared to the azobenzene derivative without calixaren. '
19
xo'
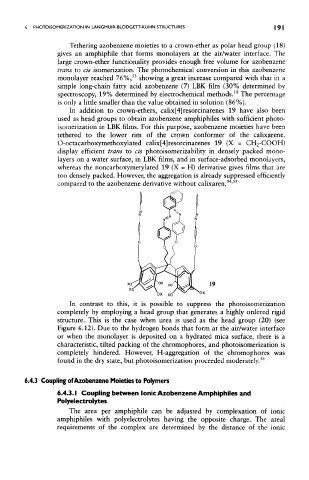
In contrast to this, it is possible to suppress the photoisomerization
completely by employing a head group that generates a highly ordered rigid
structure. This is the case when urea is used as the head group (20) (see
Figure 6.12). Due to the hydrogen bonds that form at the air/water interface
or when the monolayer is deposited on a hydrated mica surface, there is a
characteristic, tilted packing of the chromophores, and photoisomerization is
completely hindered. However, H-aggregation of the chromophores was
56
found in the dry state, but photoisomerization proceeded moderately.
6.4.3 Coupling of Azobenzene Moieties to Polymers
6.4.3.1 Coupling between Ionic Azobenzene Amphiphiles and
Pol/electrolytes
The area per amphiphile can be adjusted by complexation of ionic
amphiphiles with polyelectrolytes having the opposite charge. The areal
requirements of the complex are determined by the distance of the ionic