Page 216 - Photoreactive Organic Thin Films
P. 216
6. PHOTOISOMERIZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES If 5
o
o
o
o
0.5
0.4 0.5 0.6 0.7
30
l
Area per Molecule at 25 mN m~ /nm 2 SO,Na
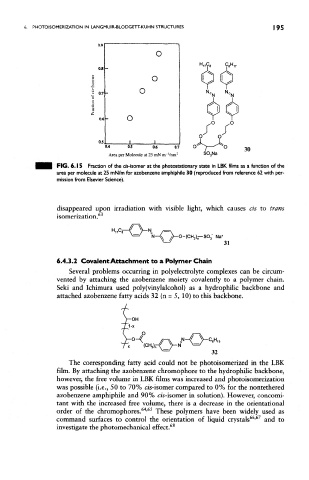
FIG. 6.15 Fraction of the ds-isorner at the photostationary state in LBK films as a function of the
area per molecule at 25 mN/m for azobenzene amphiphile 30 (reproduced from reference 62 with per-
mission from Elsevier Science).
disappeared upon irradiation with visible light, which causes cis to trans
isomerization. 63
H«C,
6.4.3.2 Covalent Attachment to a Polymer Chain
Several problems occurring in polyelectrolyte complexes can be circum-
vented by attaching the azobenzene moiety covalently to a polymer chain.
Seki and Ichimura used poly(vinylalcohol) as a hydrophilic backbone and
attached azobenzene fatty acids 32 (n = 5,10} to this backbone.
The corresponding fatty acid could not be photoisomerized in the LBK
film. By attaching the azobenzene chromophore to the hydrophilic backbone,
however, the free volume in LBK films was increased and photoisomerization
was possible (i.e., 50 to 70% ds-isomer compared to 0% for the nontethered
azobenzene amphiphile and 90% cw-isomer in solution). However, concomi-
tant with the increased free volume, there is a decrease in the orientational
64 65
order of the chromophores. ' These polymers have been widely used as
66 67
command surfaces to control the orientation of liquid crystals ' and to
investigate the photomechanical effect. 68