Page 218 - Photoreactive Organic Thin Films
P. 218
6, PHOTOISOMERIZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES | f J
homopolymer's LBK film, the chromophores are aggregated, as evidenced by
the shift in the UV-vis spectrum, but isomerization is possible. This was
attributed to the higher degree of flexibility in a liquid crystalline system as
compared to a crystalline system. Photoisomerization is accompanied by a
major change in the structure of the LBK film. A compressed monolayer
at the air/water interface with tightly packed chromophores in a liquid crys-
talline order is deposited on a solid support, giving a smectic-like multilayer
assembly. This structure is metastable and photoisomerization from trans to
cis is enough to destabilize the structure. So, upon irradiation with UV light,
the double layers are irreversibly destroyed. After a photoinduced trans-cis-
trans isomerization cycle, a largely disordered structure is obtained, with a
73
complete loss of the layered structure (see Figure 6.19). The structural
changes are accompanied by irreversible changes in the optical properties,
which can be monitored by surface plasmon microscopy.
On the other hand, in the LBK films of the copolymers 35, aggregation
does not occur because of the spatial decoupling of the chromophores.
Photoisomerization takes places readily in these films upon irradiation and
causes changes in the optical properties, which again can be probed by sur-
face plasmon spectroscopy. In this case, however, the spectra and the changes
73 75
in the optical properties are fully reversible upon alternate irradiation. '
LBK films of photochromic liquid crystalline copolymers containing
mesogenic cholesterol and azobenzene side chains attached to a potysiloxane
main chain are another example of the approach of using order and flex-
ibility of liquid crystalline phases. In the LBK films, no aggregation of the
76
chromophores is observed, and photoisomerization is possible.
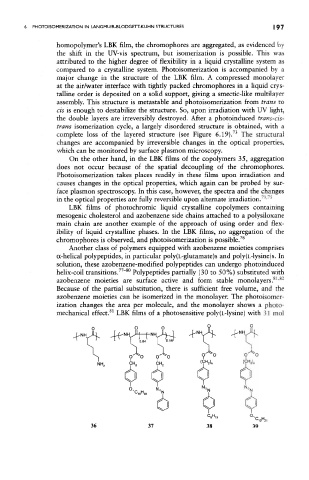
Another class of polymers equipped with azobenzene moieties comprises
a-helical polypeptides, in particular poly(L-glutamate)s and poly{L-lysine)s. In
solution, these azobenzene-modified polypeptides can undergo photoinduced
77 80
helix-coil transitions. " Polypeptides partially (30 to 50%) substituted with
81 82
azobenzene moieties are surface active and form stable monolayers. '
Because of the partial substitution, there is sufficient free volume, and the
azobenzene moieties can be isomerized in the monolayer. The photoisomer-
ization changes the area per molecule, and the monolayer shows a photo-
81
mechanical effect. LBK films of a photosensitive poly(L-lysine) with 31 mol
36 37