Page 222 - Photoreactive Organic Thin Films
P. 222
6. PHOTOISOMERIZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES 20 I
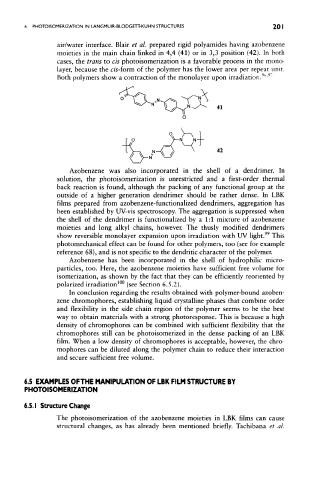
air/water interface. Blair et aL prepared rigid polyamides having azobenzene
moieties in the main chain linked in 4,4 (41) or in 3,3 position (42). In both
cases, the trans to cis photoisomerization is a favorable process in the mono-
layer, because the c/s-form of the polymer has the lower area per repeat unit.
96 9
Both polymers show a contraction of the monolayer upon irradiation. '
41
42
Azobenzene was also incorporated in the shell of a dendrimer. In
solution, the photoisomerization is unrestricted and a first-order thermal
back reaction is found, although the packing of any functional group at the
outside of a higher generation dendrimer should be rather dense. In LBK
films prepared from azobenzene-functionalized dendrimers, aggregation has
been established by UV-vis spectroscopy. The aggregation is suppressed when
the shell of the dendrimer is functionalized by a 1:1 mixture of azobenzene
moieties and long alkyl chains, however. The thusly modified dendrimers
show reversible monolayer expansion upon irradiation with UV light." This
photomechanical effect can be found for other polymers, too (see for example
reference 68), and is not specific to the dendritic character of the polymer.
Azobenzene has been incorporated in the shell of hydrophilic micro-
particles, too. Here, the azobenzene moieties have sufficient free volume for
isomerization, as shown by the fact that they can be efficiently reoriented by
100
polarized irradiation (see Section 6.5.2).
In conclusion regarding the results obtained with polymer-bound azoben-
zene chromophores, establishing liquid crystalline phases that combine order
and flexibility in the side chain region of the polymer seems to be the best
way to obtain materials with a strong photoresponse. This is because a high
density of chromophores can be combined with sufficient flexibility that the
chromophores still can be photoisomerized in the dense packing of an LBK
film. When a low density of chromophores is acceptable, however, the chro-
mophores can be diluted along the polymer chain to reduce their interaction
and secure sufficient free volume.
6.5 EXAMPLES OF THE MANIPULATION OF LBK FILM STRUCTURE BY
PHOTOISOMERI2ATION
6.5.1 Structure Change
The photoisomerization of the azobenzene moieties in LBK films can cause
structural changes, as has already been mentioned briefly. Tachibana et aL