Page 226 - Photoreactive Organic Thin Films
P. 226
6. PHOTOISOMERIZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES 205
340
0.7 CD
3L
(D
0.6 330 (Q
21.
OJ 0.5
. "0
320 o
0)
0.4
310
0.1
0.0 300
0 5 10 15 20 25 30
Irradiation Time/ min
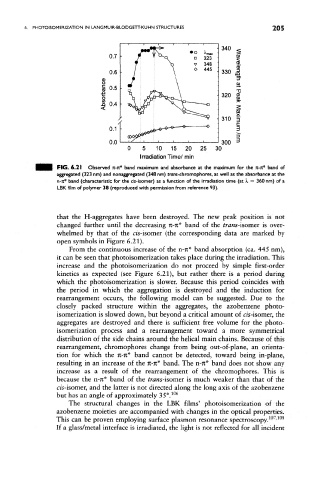
FIG. 6.2 1 Observed TC-JC* band maximum and absorbance at the maximum for the Jt-rc* band of
aggregated (323 nm) and nonaggregated (348 nm) trons-chromophores, as well as the absorbance at the
n-rc* band (characteristic for the c;s-i$omer) as a function of the irradiation time (at X =* 360 nm) of a
LBK film of polymer 38 (reproduced with permission from reference 93).
that the H-aggregates have been destroyed. The new peak position is not
changed further until the decreasing n-n* band of the fnws-isomer is over-
whelmed by that of the cis-isomer (the corresponding data are marked by
open symbols in Figure 6.21).
From the continuous increase of the n-rc* band absorption (ca. 445 nm),
it can be seen that photoisomerization takes place during the irradiation. This
increase and the photoisomerization do not proceed by simple first-order
kinetics as expected (see Figure 6.21), but rather there is a period during
which the photoisomerization is slower. Because this period coincides with
the period in which the aggregation is destroyed and the induction for
rearrangement occurs, the following model can be suggested. Due to the
closely packed structure within the aggregates, the azobenzene photo-
isomerization is slowed down, but beyond a critical amount of ds-isomer, the
aggregates are destroyed and there is sufficient free volume for the photo-
isomerization process and a rearrangement toward a more symmetrical
distribution of the side chains around the helical main chains. Because of this
rearrangement, chromophores change from being out-of-plane, an orienta-
tion for which the n-n* band cannot be detected, toward being in-plane,
resulting in an increase of the n-n* band. The n-rc* band does not show any
increase as a result of the rearrangement of the chromophores. This is
because the n-rc* band of the trans-isomer is much weaker than that of the
ds-isomer, and the latter is not directed along the long axis of the azobenzene
106
but has an angle of approximately 35°.
The structural changes in the LBK films' photoisomerization of the
azobenzene moieties are accompanied with changes in the optical properties.
107 108
This can be proven employing surface plasmon resonance spectroscopy. '
If a glass/metal interface is irradiated, the light is not reflected for all incident