Page 213 - Photoreactive Organic Thin Films
P. 213
192 HENN1NG MENZEL
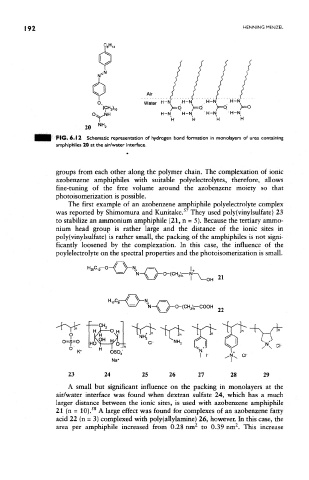
Air
Water H-N , H-N H-N H-N
)=0 >=Q /=0 >=0
H-N 'H-N H-N -H-N
H H H H
PIG. 6.12 Schematic representation of hydrogen bond formation in monolayers of urea containing
amphiphiles 20 at the air/water interface.
groups from each other along the polymer chain. The compiexation of ionic
azobenzene amphiphiles with suitable polyelectrolytes, therefore, allows
fine-tuning of the free volume around the azobenzene moiety so that
photoisomerization is possible.
The first example of an azobenzene amphiphile polyelectrolyte complex
57
was reported by Shimomura and Kunitake. They used poly(vinylsulfate) 23
to stabilize an ammonium amphiphile (21, n = 5). Because the tertiary ammo-
nium head group is rather large and the distance of the ionic sites in
poly(vinylsulfate) is rather small, the packing of the amphiphiles is not signi-
ficantly loosened by the compiexation. In this case, the influence of the
poylelectrolyte on the spectral properties and the photoisomerization is small.
0-(CH 2) n— N-
-OH 21
23 24 25 26 27 28 29
A small but significant influence on the packing in monolayers at the
air/water interface was found when dextran sulfate 24, which has a much
larger distance between the ionic sites, is used with azobenzene amphiphile
58
21 (n = 10). A large effect was found for complexes of an azobenzene fatty
acid 22 (n = 3) complexed with poly(allylamine) 26, however. In this case, the
2 2
area per amphiphile increased from 0.28 nm to 0.39 nm . This increase