Page 208 - Photoreactive Organic Thin Films
P. 208
6. PHOTOISOMERIZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES
trans isomerization was not hindered. This is due to the fact that ds-isomers
2 3 34
have higher areal requirements than fraws-isomers. ' '
LBK films of the azobenzene containing fatty acid 7 show a similar
behavior. When compressed and transferred in the fraws-form, a peak shift
due to aggregation is observed and the photoisomerization is hindered. The
chromophores are very densely packed, as established by STM measure-
35
ments, When compressed and deposited in the ds-form, i.e., under illumina-
tion with UV light, there is no aggregation, and the cis to trans isomerization
is unrestricted. Alternate irradiation under constant surface area causes
36
changes in the surface pressure.
The time necessary to reach the photostationary ds-state for the first time
in LBK films of this azobenzene amphiphile transferred in the trans-statt is
37
considerably longer than for the subsequent cycles. This is explained by the
time it takes to break up some aggregates in the dense packing of the LBK
35 17
film. The extent of isomerization, determined by Brode's method, is given
to be 30%. Reestimation employing electrochemical methods showed that
18
the irradiation yielded a ds-isomer count of only 19%.
The extent of the isomerization hindrance depends on the structure of the
azobenzene amphiphile. When the azobenzene is located in the middle of
the alkyl chain (i.e., 7), the trans to cis photoisomerization in LBK films is
17 18
severely hindered but not completely suppressed. ' However, when the
azobenzene moiety is located directly at the head group, as in 12, the isomer-
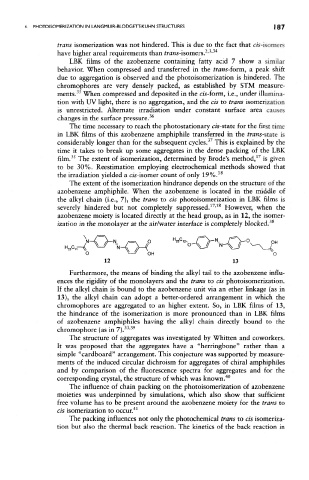
ization in the monolayer at the air/water interface is completely blocked. 38
Furthermore, the means of binding the alkyl tail to the azobenzene influ-
ences the rigidity of the monolayers and the trans to cis photoisomerization.
If the alkyl chain is bound to the azobenzene unit via an ether linkage (as in
13), the alkyl chain can adopt a better-ordered arrangement in which the
chromophores are aggregated to an higher extent. So, in LBK films of 13,
the hindrance of the isomerization is more pronounced than in LBK films
of azobenzene amphiphiles having the alkyl chain directly bound to the
33 39
chromophore (as in 7). '
The structure of aggregates was investigated by Whitten and coworkers.
It was proposed that the aggregates have a "herringbone" rather than a
simple "cardboard" arrangement. This conjecture was supported by measure-
ments of the induced circular dichroism for aggregates of chiral amphiphiles
and by comparison of the fluorescence spectra for aggregates and for the
corresponding crystal, the structure of which was known. 40
The influence of chain packing on the photoisomerization of azobenzene
moieties was underpinned by simulations, which also show that sufficient
free volume has to be present around the azobenzene moiety for the trans to
cis isomerization to occur. 41
The packing influences not only the photochemical trans to ds isomeriza-
tion but also the thermal back reaction. The kinetics of the back reaction in