Page 204 - Photoreactive Organic Thin Films
P. 204
6. PHOTOISOMERiZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES 183
OMe
OMe
OMe
OMe
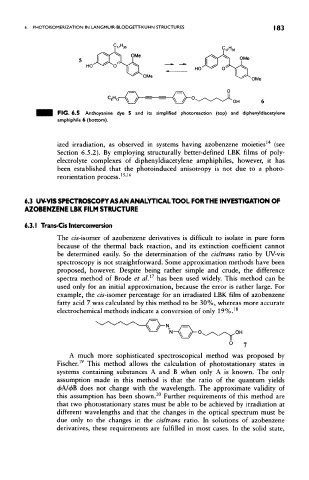
FIG. 6.5 Anthoyanine dye 5 and its simplified photoreaction (top) and diphenyldiacetytene
amphiphile 6 (bottom).
14
ized irradiation, as observed in systems having azobenzene moieties (see
Section 6.5.2). By employing structurally better-defined LBK films of poly-
electrolyte complexes of diphenyldiacetylene amphiphiles, however, it has
been established that the photoinduced anisotropy is not due to a photo-
15 16
reorientation process. '
6.3 UV-VIS SPECTROSCOPY AS AN ANALYTICAL TOOL FORTHE INVESTIGATION OF
AZOBENZENE LBK FILM STRUCTURE
6.3.1 Trans-Cis Interconversion
The c/s-isomer of azobenzene derivatives is difficult to isolate in pure form
because of the thermal back reaction, and its extinction coefficient cannot
be determined easily. So the determination of the dsltrans ratio by UV-vis
spectroscopy is not straightforward. Some approximation methods have been
proposed, however. Despite being rather simple and crude, the difference
17
spectra method of Erode et al. has been used widely. This method can be
used only for an initial approximation, because the error is rather large. For
example, the ds-isomer percentage for an irradiated LBK film of azobenzene
fatty acid 7 was calculated by this method to be 30%, whereas more accurate
18
electrochemical methods indicate a conversion of only 19%.
A much more sophisticated spectroscopical method was proposed by
19
Fischer. This method allows the calculation of photostationary states in
systems containing substances A and B when only A is known. The only
assumption made in this method is that the ratio of the quantum yields
<£A/<£B does not change with the wavelength. The approximate validity of
20
this assumption has been shown. Further requirements of this method are
that two photostationary states must be able to be achieved by irradiation at
different wavelengths and that the changes in the optical spectrum must be
due only to the changes in the cis/trans ratio. In solutions of azobenzene
derivatives, these requirements are fulfilled in most cases. In the solid state,