Page 202 - Photoreactive Organic Thin Films
P. 202
6. PHQTOISOMERIZATSON IN LANGMUIR-BLODGETT-KUHN STRUCTURES 181
R-O
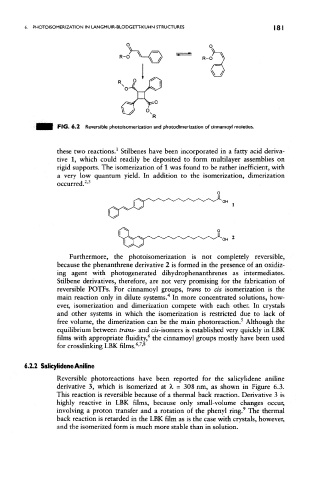
FIG. 6.2 Reversible photoisomerization and photodimerization of cinnamoyl moieties.
1
these two reactions. Stilbenes have been incorporated in a fatty acid deriva-
tive 1, which could readily be deposited to form multilayer assemblies on
rigid supports. The isomerization of 1 was found to be rather inefficient, with
a very low quantum yield. In addition to the isomerization, dimerization
2 3
occurred. '
OH
Furthermore, the photoisomerization is not completely reversible,
because the phenanthrene derivative 2 is formed in the presence of an oxidiz-
ing agent with photogenerated dihydrophenanthrenes as intermediates,
Stilbene derivatives, therefore, are not very promising for the fabrication of
reversible POTFs. For cinnamoyl groups, trans to ds isomerization is the
4
main reaction only in dilute systems. In more concentrated solutions, how-
ever, isomerization and dimerization compete with each other. In crystals
and other systems in which the isomerization is restricted due to lack of
5
free volume, the dimerization can be the main photoreaction. Although the
equilibrium between trans- and as-isomers is established very quickly in LBK
6
films with appropriate fluidity, the cinnamoyl groups mostly have been used
6 7 8
for crosslinking LBK films. ' '
6.2.2 Salicylidene Aniline
Reversible photoreactions have been reported for the salicylidene aniline
derivative 3, which is isomerized at K = 308 nm, as shown in Figure 6,3.
This reaction is reversible because of a thermal back reaction. Derivative 3 is
highly reactive in LBK films, because only small-volume changes occur,
9
involving a proton transfer and a rotation of the phenyl ring. The thermal
back reaction is retarded in the LBK film as is the case with crystals, however,
and the isomerized form is much more stable than in solution.