Page 214 - Photoreactive Organic Thin Films
P. 214
6. PHOTOISOMERIZATION IN LANGMUIR-BLODGETT-KUHN STRUCTURES 193
0.7
0,6
0.5
0.4
0.3
10 11
PH
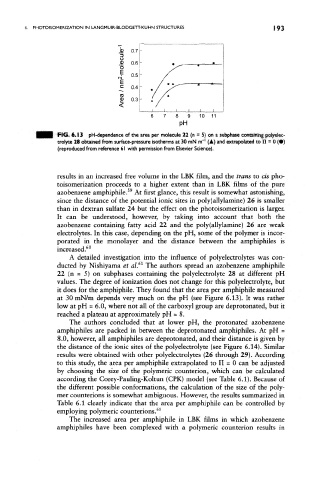
FIG. 6.13 pH-dependence of the area per molecule 22 (n = 5) on a subphase containing poiyeiec-
trolyte 28 obtained from surface-pressure isotherms at 30 mN nrf' (A) and extrapolated to II = 0 (•)
(reproduced from reference 61 with permission from Elsevier Science).
results in an increased free volume in the LBK film, and the trans to cts pho-
toisomerization proceeds to a higher extent than in LBK films of the pure
59
azobenzene amphiphile. At first glance, this result is somewhat astonishing,
since the distance of the potential ionic sites in poly(aliyiamine) 26 is smaller
than in dextran sulfate 24 but the effect on the photoisomerization is larger.
It can be understood, however, by taking into account that both the
azobenzene containing fatty acid 22 and the poly(allylamine) 26 are weak
electrolytes. In this case, depending on the pH, some of the polymer is incor-
porated in the monolayer and the distance between the amphiphiles is
increased. 60
A detailed investigation into the influence of polyelectrolytes was con-
61
ducted by Nishiyama et al, The authors spread an azobenzene amphiphile
22 (n = 5) on subphases containing the polyelectrolyte 28 at different pH
values. The degree of ionization does not change for this polyelectrolyte, but
it does for the amphiphile. They found that the area per amphiphile measured
at 30 mN/m depends very much on the pH (see Figure 6.13). It was rather
low at pH = 6.0, where not all of the carboxyl group are deprotonated, but it
reached a plateau at approximately pH = 8.
The authors concluded that at lower pH, the protonated azobenzene
amphiphiles are packed in between the deprotonated amphiphiles. At pH =
8.0, however, all amphiphiles are deprotonated, and their distance is given by
the distance of the ionic sites of the polyelectrolyte (see Figure 6.14). Similar
results were obtained with other polyelectrolytes (26 through 29). According
to this study, the area per amphiphile extrapolated to II = 0 can be adjusted
by choosing the size of the polymeric counterion, which can be calculated
according the Corey-Pauling-Koltun (CPK) model (see Table 6.1). Because of
the different possible conformations, the calculation of the size of the poly-
mer counterions is somewhat ambiguous. However, the results summarized in
Table 6.1 clearly indicate that the area per amphiphile can be controlled by
employing polymeric counterions. 61
The increased area per amphiphile in LBK films in which azobenzene
amphiphiles have been complexed with a polymeric counterion results in