Page 244 - Photoreactive Organic Thin Films
P. 244
7, ELECTRONIC AND OPTICAL TRANSDUCTION OF PHOTISOMERIZATION PROCESSES 223
305 nm
<X<
320 nm
430 nm
10
5H
_ 1 a (trans)
-10-
1b(ana)
-15
-0.8 -0.6 -0.4 -0.2
E/V(vsSCE)
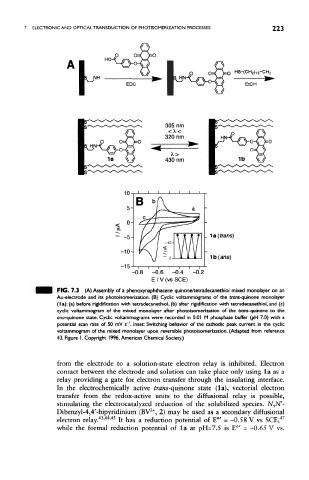
FIG. 7.3 (A) Assembly of a phenoxynaphthacene quinone/tetradecanethiol mixed monolayer on an
Au-electrode and its photoisomerization. (B) Cyclic voltammograms of the trans-quinone monolayer
(la): (a) before rigidiflcation with tetradecanethiol, (b) after rigidiflcation with tetradecanethiol, and (c)
cyclic voltammogram of the mixed monolayer after photoisomerization of the trans-quinone to the
ono-quinone state. Cyclic voltammograms were recorded in 0.01 M phosphate buffer (pH 7,0) with a
potential scan rate of 50 mV s~'. Inset: Switching behavior of the cathodic peak current in the cyclic
voltammogram of the mixed monolayer upon reversible photoisomerization. (Adapted from reference
43, Figure I.Copyright 1996, American Chemical Society.)
from the electrode to a solution-state electron relay is inhibited. Electron
contact between the electrode and solution can take place only using la as a
relay providing a gate for electron transfer through the insulating interface.
In the electrochemically active ifnws-quinone state (la), vectorial electron
transfer from the redox-active units to the diffusional relay is possible,
stimulating the electrocatalyzed reduction of the solubilized species. N,N'-
2
Dibenzyl-4,4'-bipyridinium (BV % 2) may be used as a secondary diffusional
43 44 45
electron relay. ' ' It has a reduction potential of E°' = -0.58 V vs SCE, 47
while the formal reduction potential of la at pH=7.5 is E°' = -0.65 V vs.