Page 247 - Photoreactive Organic Thin Films
P. 247
226 EUGENIi KATZ.ANDREW N. SHIPWAY,AND ITAMARWiLlNER
B
0.4 -0.2 0 0.2 0.4
E / V (vs. SCE)
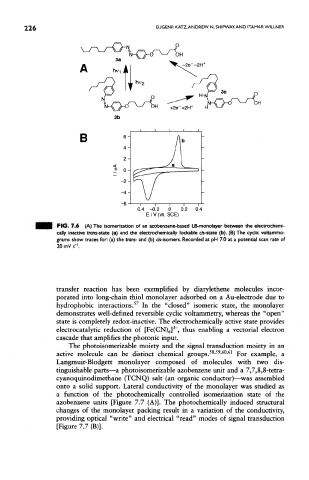
FIG. 7.6 (A) The isomerization of an azobenzene-based LB-monolayer between the electrochemi-
cally inactive trans-state (a) and the electrochemically lockable as-state (b). (B) The cyclic voltamrno-
grams show traces for: (a) the trans- and (b) cre-isomers. Recorded at pH 7.0 at a potential scan rate of
20 mV s-'.
transfer reaction has been exemplified by diarylethene molecules incor-
porated into long-chain thiol monolayer adsorbed on a Au-electrode due to
57
hydrophobic interactions. In the "closed" isomeric state, the monolayer
demonstrates well-defined reversible cyclic voltammetry, whereas the "open"
state is completely redox-inactive. The electrochemically active state provides
3
electrocatalytic reduction of [Fe(CN) 6] ~, thus enabling a vectorial electron
cascade that amplifies the photonic input.
The photoisomerizable moiety and the signal transduction moiety in an
58 59 60 61
active molecule can be distinct chemical groups. ' ' ' For example, a
Langmuir-Blodgett monolayer composed of molecules with two dis-
tinguishable parts—a photoisomerizable azobenzene unit and a 7,7,8,8-tetra-
cyanoquinodimethane (TCNQ) salt (an organic conductor)—was assembled
onto a solid support. Lateral conductivity of the monolayer was studied as
a function of the photochemically controlled isomerization state of the
azobenzene units [Figure 7.7 (A)]. The photochemically induced structural
changes of the monolayer packing result in a variation of the conductivity,
providing optical "write" and electrical "read" modes of signal transduction
[Figure 7.7 (B)].