Page 246 - Photoreactive Organic Thin Films
P. 246
7. ELECTRONIC AND OPTICAL TRANSDUCTION OF PHOTISOMERIZATION PROCESSES 225
-20-
-0.4
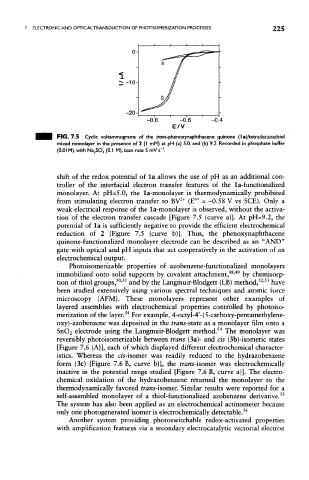
FIG. 7.5 Cyclic voltammograms of the trans-phenoxynaphthacene quinone (la)/tetradecanethiol
mixed monolayer in the presence of 2 (I mM) at pH (a) 5.0, and (b) 9.2. Recorded in phosphate buffer
1
(0.01M),with Na 2SO 4 (O.I M),scan rate 5 mV s' .
shift of the redox potential of la allows the use of pH as an additional con-
troller of the interfacial electron transfer features of the la-functionalized
monolayer. At pH=5.0, the la-monolayer is thermodynamically prohibited
0/
2
from stimulating electron transfer to BV * (E = -0.58 V vs SCE). Only a
weak electrical response of the la-monolayer is observed, without the activa-
tion of the electron transfer cascade [Figure 7.5 (curve a)]. At pH=9.2, the
potential of la is sufficiently negative to provide the efficient electrochemical
reduction of 2 [Figure 7.5 (curve b)]. Thus, the phenoxynaphthacene
quinone-functionalized monolayer electrode can be described as an "AND"
gate with optical and pH inputs that act cooperatively in the activation of an
electrochemical output.
Photoisomerizable properties of azobenzene-functionalized monolayers
48 49
immobilized onto solid supports by covalent attachment, ' by chemisorp-
50 51 52 53
tion of thiol groups, ' and by the Langmuir-Blodgett (LB) method, * have
been studied extensively using various spectral techniques and atomic force
microscopy (AFM). These monolayers represent other examples of
layered assemblies with electrochemical properties controlled by photoiso-
54 /
merization of the layer. For example, 4-octyl-4 -(5-carboxy-pentamethylene-
oxy)-azobenzene was deposited in the ^raws-state as a monolayer film onto a
54
SnO 2 electrode using the Langmuir-Blodgett method. The monolayer was
reversibly photoisomerizable between trans (3a)~ and cis (3b)-isomeric states
[Figure 7.6 (A)], each of which displayed different electrochemical character-
istics. Whereas the ds-isomer was readily reduced to the hydrazobenzene
form (3c) [Figure 7.6 B, curve b)], the £ra«s-isomer was electrochemically
inactive in the potential range studied [Figure 7.6 B, curve a)]. The electro-
chemical oxidation of the hydrazobenzene returned the monolayer to the
thermodynamically favored fnws-isomer. Similar results were reported for a
5
self-assembled monolayer of a thiol-functionalized azobenzene derivative. ^
The system has also been applied as an electrochemical actinometer because
only one photogenerated isomer is electrochemically detectable. 56
Another system providing photoswitchable redox-activated properties
with amplification features via a secondary electrocatalytic vectorial electron