Page 245 - Photoreactive Organic Thin Films
P. 245
224 EUGENII KATZ.ANDREW N. SHIPWAY.AND ITAMARWILLNER
-10- -1 a (trans)
305 nm
<K<
430 nm IX 320 nm -15-
ib(ana)
-20
-0.8 -0.6 -0,4
E/V(vs SCE)
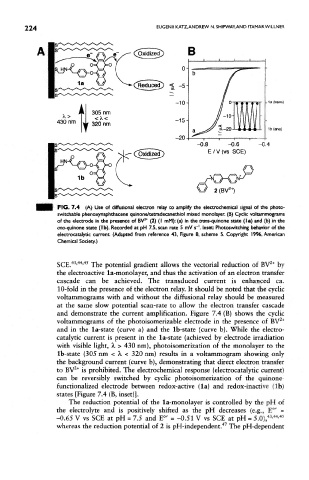
FIG. 7.4 (A) Use of diffusional electron relay to amplify the electrochemical signal of the photo-
switchable phenoxynaphthacene quinone/tetradecanethiol mixed monolayer. (B) Cyclic voltammograms
2
of the electrode in the presence of BV * (2) (I mM): (a) in the trans-quinone state (la) and (b) in the
ono-quinone state (Ib). Recorded at pH 7.5, scan rate 5 mV s~'. Inset Photoswitching behavior of the
electrocatalytic current (Adapted from reference 43, Figure 8, scheme 5. Copyright 1996, American
Chemical Society.)
43 44 45 2+
SCE. ' ' The potential gradient allows the vectorial reduction of BV by
the electroactive la-monolayer, and thus the activation of an electron transfer
cascade can be achieved. The transduced current is enhanced ca.
10-fold in the presence of the electron relay. It should be noted that the cyclic
voltammograms with and without the diffusional relay should be measured
at the same slow potential scan-rate to allow the electron transfer cascade
and demonstrate the current amplification. Figure 7.4 (B) shows the cyclic
2+
voltammograms of the photoisomerizable electrode in the presence of BV
and in the la-state (curve a) and the Ib-state (curve b). While the electro-
catalytic current is present in the la-state (achieved by electrode irradiation
with visible light, X > 430 nm), photoisomerization of the monolayer to the
Ib-state (305 nrn < A, < 320 nm) results in a voltammogram showing only
the background current (curve b), demonstrating that direct electron transfer
2+
to BV is prohibited. The electrochemical response (electrocatalytic current)
can be reversibly switched by cyclic photoisomerization of the quinone-
functionalized electrode between redox-active (la) and redox-inactive (Ib)
states [Figure 7.4 (B, inset)].
The reduction potential of the la-monolayer is controlled by the pH of
0/
the electrolyte and is positively shifted as the pH decreases (e.g., E =
43 44 45
-0.65 V vs SCE at pH = 7.5 and E°' = -0.51 V vs SCE at pH = 5.0), ' '
47
whereas the reduction potential of 2 is pH-independent. The pH-dependent