Page 287 - Physical chemistry understanding our chemical world
P. 287
254 ACIDS AND BASES
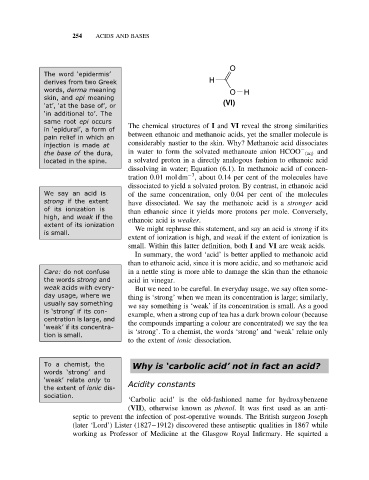
O
The word ‘epidermis’
derives from two Greek H
words, derma meaning O H
skin, and epi meaning
‘at’, ‘at the base of’, or (VI)
‘in additional to’. The
same root epi occurs The chemical structures of I and VI reveal the strong similarities
in ‘epidural’, a form of
pain relief in which an between ethanoic and methanoic acids, yet the smaller molecule is
injection is made at considerably nastier to the skin. Why? Methanoic acid dissociates
−
the base of the dura, in water to form the solvated methanoate anion HCOO (aq) and
located in the spine. a solvated proton in a directly analogous fashion to ethanoic acid
dissolving in water; Equation (6.1). In methanoic acid of concen-
−3
tration 0.01 mol dm , about 0.14 per cent of the molecules have
dissociated to yield a solvated proton. By contrast, in ethanoic acid
We say an acid is of the same concentration, only 0.04 per cent of the molecules
strong if the extent have dissociated. We say the methanoic acid is a stronger acid
of its ionization is than ethanoic since it yields more protons per mole. Conversely,
high, and weak if the ethanoic acid is weaker.
extent of its ionization
We might rephrase this statement, and say an acid is strong if its
is small.
extent of ionization is high, and weak if the extent of ionization is
small. Within this latter definition, both I and VI are weak acids.
In summary, the word ‘acid’ is better applied to methanoic acid
than to ethanoic acid, since it is more acidic, and so methanoic acid
Care: do not confuse in a nettle sting is more able to damage the skin than the ethanoic
the words strong and acid in vinegar.
weak acids with every- But we need to be careful. In everyday usage, we say often some-
day usage, where we thing is ‘strong’ when we mean its concentration is large; similarly,
usually say something
we say something is ‘weak’ if its concentration is small. As a good
is ‘strong’ if its con- example, when a strong cup of tea has a dark brown colour (because
centration is large, and
the compounds imparting a colour are concentrated) we say the tea
‘weak’ if its concentra-
tion is small. is ‘strong’. To a chemist, the words ‘strong’ and ‘weak’ relate only
to the extent of ionic dissociation.
To a chemist, the Why is ‘carbolic acid’ not in fact an acid?
words ‘strong’ and
‘weak’ relate only to Acidity constants
the extent of ionic dis-
sociation.
‘Carbolic acid’ is the old-fashioned name for hydroxybenzene
(VII), otherwise known as phenol. It was first used as an anti-
septic to prevent the infection of post-operative wounds. The British surgeon Joseph
(later ‘Lord’) Lister (1827–1912) discovered these antiseptic qualities in 1867 while
working as Professor of Medicine at the Glasgow Royal Infirmary. He squirted a