Page 290 - Physical chemistry understanding our chemical world
P. 290
‘STRONG’ AND ‘WEAK’ ACIDS AND BASES 257
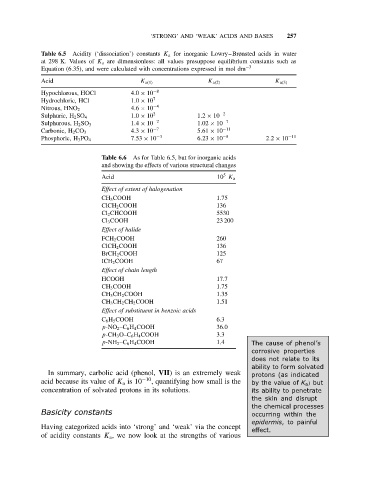
Table 6.5 Acidity (‘dissociation’) constants K a for inorganic Lowry–Brønsted acids in water
at 298 K. Values of K a are dimensionless: all values presuppose equilibrium constants such as
Equation (6.35), and were calculated with concentrations expressed in mol dm −3
Acid K a(1) K a(2) K a(3)
Hypochlorous, HOCl 4.0 × 10 −8
Hydrochloric, HCl 1.0 × 10 7
4.6 × 10 −4
Nitrous, HNO 2
1.0 × 10 2 1.2 × 10 −2
Sulphuric, H 2 SO 4
1.4 × 10 −2 1.02 × 10 −7
Sulphurous, H 2 SO 3
4.3 × 10 −7 5.61 × 10 −11
Carbonic, H 2 CO 3
Phosphoric, H 3 PO 4 7.53 × 10 −3 6.23 × 10 −8 2.2 × 10 −11
Table 6.6 As for Table 6.5, but for inorganic acids
and showing the effects of various structural changes
5
Acid 10 K a
Effect of extent of halogenation
CH 3 COOH 1.75
ClCH 2 COOH 136
Cl 2 CHCOOH 5530
Cl 3 COOH 23 200
Effect of halide
FCH 2 COOH 260
ClCH 2 COOH 136
BrCH 2 COOH 125
ICH 2 COOH 67
Effect of chain length
HCOOH 17.7
CH 3 COOH 1.75
CH 3 CH 2 COOH 1.35
CH 3 CH 2 CH 2 COOH 1.51
Effect of substituent in benzoic acids
C 6 H 5 COOH 6.3
p-NO 2 –C 6 H 4 COOH 36.0
p-CH 3 O–C 6 H 4 COOH 3.3
p-NH 2 –C 6 H 4 COOH 1.4 The cause of phenol’s
corrosive properties
does not relate to its
ability to form solvated
In summary, carbolic acid (phenol, VII) is an extremely weak protons (as indicated
acid because its value of K a is 10 −10 , quantifying how small is the by the value of K a ) but
concentration of solvated protons in its solutions. its ability to penetrate
the skin and disrupt
the chemical processes
Basicity constants occurring within the
epidermis, to painful
Having categorized acids into ‘strong’ and ‘weak’ via the concept
effect.
of acidity constants K a , we now look at the strengths of various