Page 293 - Physical chemistry understanding our chemical world
P. 293
260 ACIDS AND BASES
The equilibrium constant for the dissociation of H 2 SO 4 :
The subscript (1) tells
+
−
us we are considering [HSO ][H ]
4
the first proton to be K a(1) = [H 2 SO 4 ]
lost.
Similarly,
+
2−
[SO ][H ]
4
K a(1) is always bigger K a(2) = [HSO 4 ]
−
than K a(2) .
In fact, we can also extend this treatment to bases, looking at
the step-base addition of protons.
SAQ 6.10 The tetra-protic acid H 4 EDTA (V) has four possible proton
equilibrium constants. Write an expression for each, for K a(1) to K a(4) .
Why is an organic acid such as trichloroethanoic acid
so strong?
Effect of structure on the K a of a weak acid
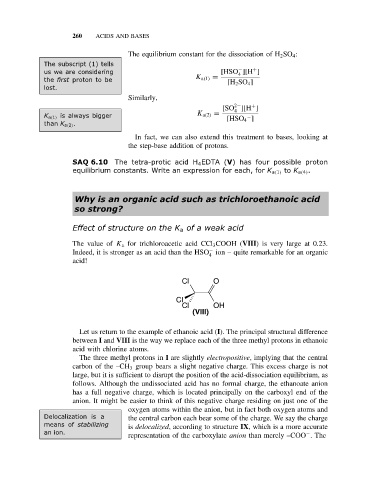
The value of K a for trichloroacetic acid CCl 3 COOH (VIII) is very large at 0.23.
−
Indeed, it is stronger as an acid than the HSO ion – quite remarkable for an organic
4
acid!
Cl O
Cl
Cl OH
(VIII)
Let us return to the example of ethanoic acid (I). The principal structural difference
between I and VIII is the way we replace each of the three methyl protons in ethanoic
acid with chlorine atoms.
The three methyl protons in I are slightly electropositive, implying that the central
carbon of the –CH 3 group bears a slight negative charge. This excess charge is not
large, but it is sufficient to disrupt the position of the acid-dissociation equilibrium, as
follows. Although the undissociated acid has no formal charge, the ethanoate anion
has a full negative charge, which is located principally on the carboxyl end of the
anion. It might be easier to think of this negative charge residing on just one of the
oxygen atoms within the anion, but in fact both oxygen atoms and
Delocalization is a the central carbon each bear some of the charge. We say the charge
means of stabilizing is delocalized, according to structure IX, which is a more accurate
an ion. representation of the carboxylate anion than merely –COO .The
−