Page 294 - Physical chemistry understanding our chemical world
P. 294
TITRATION ANALYSES 261
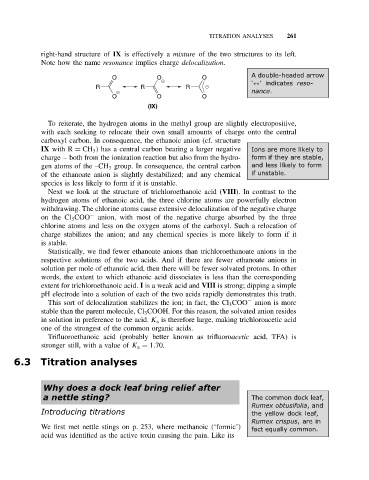
right-hand structure of IX is effectively a mixture of the two structures to its left.
Note how the name resonance implies charge delocalization.
A double-headed arrow
O O O
‘↔’indicates reso-
R R R
nance.
O O O
(IX)
To reiterate, the hydrogen atoms in the methyl group are slightly electropositive,
with each seeking to relocate their own small amounts of charge onto the central
carboxyl carbon. In consequence, the ethanoic anion (cf. structure
IX with R = CH 3 ) has a central carbon bearing a larger negative Ions are more likely to
charge – both from the ionization reaction but also from the hydro- form if they are stable,
gen atoms of the –CH 3 group. In consequence, the central carbon and less likely to form
of the ethanoate anion is slightly destabilized; and any chemical if unstable.
species is less likely to form if it is unstable.
Next we look at the structure of trichloroethanoic acid (VIII). In contrast to the
hydrogen atoms of ethanoic acid, the three chlorine atoms are powerfully electron
withdrawing. The chlorine atoms cause extensive delocalization of the negative charge
on the Cl 3 COO − anion, with most of the negative charge absorbed by the three
chlorine atoms and less on the oxygen atoms of the carboxyl. Such a relocation of
charge stabilizes the anion; and any chemical species is more likely to form if it
is stable.
Statistically, we find fewer ethanoate anions than trichloroethanoate anions in the
respective solutions of the two acids. And if there are fewer ethanoate anions in
solution per mole of ethanoic acid, then there will be fewer solvated protons. In other
words, the extent to which ethanoic acid dissociates is less than the corresponding
extent for trichloroethanoic acid. I is a weak acid and VIII is strong; dipping a simple
pH electrode into a solution of each of the two acids rapidly demonstrates this truth.
−
This sort of delocalization stabilizes the ion; in fact, the Cl 3 COO anion is more
stable than the parent molecule, Cl 3 COOH. For this reason, the solvated anion resides
in solution in preference to the acid. K a is therefore large, making trichloroacetic acid
one of the strongest of the common organic acids.
Trifluoroethanoic acid (probably better known as trifluoroacetic acid, TFA) is
stronger still, with a value of K a = 1.70.
6.3 Titration analyses
Why does a dock leaf bring relief after
a nettle sting? The common dock leaf,
Rumex obtusifolia,and
Introducing titrations the yellow dock leaf,
Rumex crispus,are in
We first met nettle stings on p. 253, where methanoic (‘formic’) fact equally common.
acid was identified as the active toxin causing the pain. Like its