Page 351 - Physical chemistry understanding our chemical world
P. 351
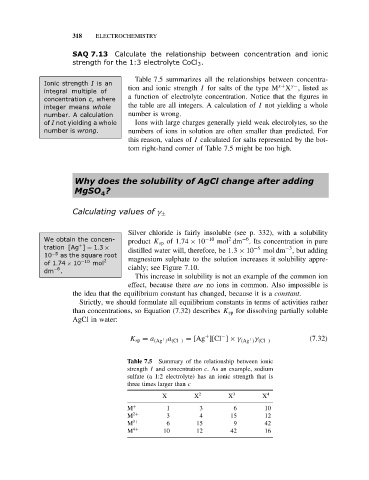
318 ELECTROCHEMISTRY
SAQ 7.13 Calculate the relationship between concentration and ionic
strength for the 1:3 electrolyte CoCl 3 .
Table 7.5 summarizes all the relationships between concentra-
Ionic strength I is an x+ y−
integral multiple of tion and ionic strength I for salts of the type M X , listed as
a function of electrolyte concentration. Notice that the figures in
concentration c,where
integer means whole the table are all integers. A calculation of I not yielding a whole
number. A calculation number is wrong.
of I not yielding a whole Ions with large charges generally yield weak electrolytes, so the
number is wrong. numbers of ions in solution are often smaller than predicted. For
this reason, values of I calculated for salts represented by the bot-
tom right-hand corner of Table 7.5 might be too high.
Why does the solubility of AgCl change after adding
MgSO ?
4
Calculating values of γ ±
Silver chloride is fairly insoluble (see p. 332), with a solubility
We obtain the concen- product K sp of 1.74 × 10 −10 mol dm . Its concentration in pure
−6
2
+
−3
tration [Ag ] = 1.3 × distilled water will, therefore, be 1.3 × 10 −5 mol dm , but adding
10 −5 as the square root magnesium sulphate to the solution increases it solubility appre-
of 1.74 × 10 −10 mol 2
dm −6 . ciably; see Figure 7.10.
This increase in solubility is not an example of the common ion
effect, because there are no ions in common. Also impossible is
the idea that the equilibrium constant has changed, because it is a constant.
Strictly, we should formulate all equilibrium constants in terms of activities rather
than concentrations, so Equation (7.32) describes K sp for dissolving partially soluble
AgCl in water:
+ γ
− = [Ag ][Cl ] × γ
+ a
K sp = a (Ag ) (Cl ) + − (Ag ) (Cl ) (7.32)
−
Table 7.5 Summary of the relationship between ionic
strength I and concentration c. As an example, sodium
sulfate (a 1:2 electrolyte) has an ionic strength that is
three times larger than c
X − X 2− X 3− X 4−
M + 1 3 6 10
M 2+ 3 4 15 12
M 3+ 6 15 9 42
M 4+ 10 12 42 16