Page 360 - Physical chemistry understanding our chemical world
P. 360
HALF-CELLS AND THE NERNST EQUATION 327
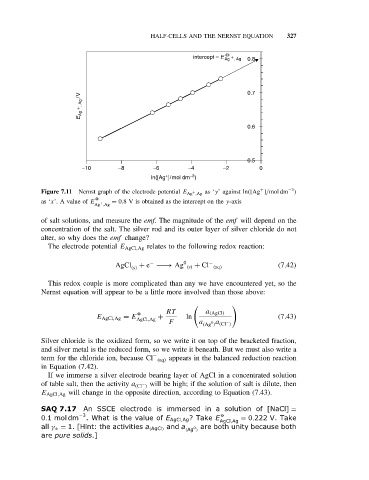
intercept = E Ag + , Ag 0.8
E Ag + , Ag /V 0.7
0.6
0.5
−10 −8 −6 −4 −2 0
+
−3
ln([Ag ]/mol dm )
−3
+
Figure 7.11 Nernst graph of the electrode potential E Ag ,Ag as ‘y’ against ln([Ag ]/mol dm )
+
O
as ‘x’. A value of E = 0.8 V is obtained as the intercept on the y-axis
+
Ag ,Ag
of salt solutions, and measure the emf. The magnitude of the emf will depend on the
concentration of the salt. The silver rod and its outer layer of silver chloride do not
alter, so why does the emf change?
The electrode potential E AgCl,Ag relates to the following redox reaction:
0
AgCl + e −−→ Ag (s) + Cl (aq) (7.42)
−
−
(s)
This redox couple is more complicated than any we have encountered yet, so the
Nernst equation will appear to be a little more involved than those above:
RT a (AgCl)
O
E AgCl,Ag = E AgCl,Ag + ln (7.43)
F a (Ag ) (Cl )
0 a
−
Silver chloride is the oxidized form, so we write it on top of the bracketed fraction,
and silver metal is the reduced form, so we write it beneath. But we must also write a
term for the chloride ion, because Cl (aq) appears in the balanced reduction reaction
−
in Equation (7.42).
If we immerse a silver electrode bearing layer of AgCl in a concentrated solution
− will be high; if the solution of salt is dilute, then
of table salt, then the activity a (Cl )
E AgCl,Ag will change in the opposite direction, according to Equation (7.43).
SAQ 7.17 An SSCE electrode is immersed in a solution of [NaCl] =
O
0.1mol dm −3 . What is the value of E AgCl,Ag ?Take E AgCl,Ag = 0.222 V. Take
all γ ± = 1. [Hint: the activities a (AgCl) and a 0 are both unity because both
(Ag )
are pure solids.]