Page 365 - Physical chemistry understanding our chemical world
P. 365
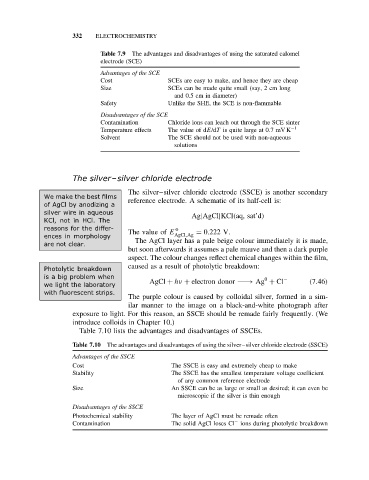
332 ELECTROCHEMISTRY
Table 7.9 The advantages and disadvantages of using the saturated calomel
electrode (SCE)
Advantages of the SCE
Cost SCEs are easy to make, and hence they are cheap
Size SCEs can be made quite small (say, 2 cm long
and 0.5 cm in diameter)
Safety Unlike the SHE, the SCE is non-flammable
Disadvantages of the SCE
Contamination Chloride ions can leach out through the SCE sinter
Temperature effects The value of dE/dT is quite large at 0.7mV K −1
Solvent The SCE should not be used with non-aqueous
solutions
The silver–silver chloride electrode
The silver–silver chloride electrode (SSCE) is another secondary
We make the best films reference electrode. A schematic of its half-cell is:
of AgCl by anodizing a
silver wire in aqueous
Ag|AgCl|KCl(aq, sat’d)
KCl, not in HCl. The
reasons for the differ- O
The value of E = 0.222 V.
ences in morphology AgCl,Ag
are not clear. The AgCl layer has a pale beige colour immediately it is made,
but soon afterwards it assumes a pale mauve and then a dark purple
aspect. The colour changes reflect chemical changes within the film,
Photolytic breakdown caused as a result of photolytic breakdown:
is a big problem when 0 −
we light the laboratory AgCl + hν + electron donor −−→ Ag + Cl (7.46)
with fluorescent strips.
The purple colour is caused by colloidal silver, formed in a sim-
ilar manner to the image on a black-and-white photograph after
exposure to light. For this reason, an SSCE should be remade fairly frequently. (We
introduce colloids in Chapter 10.)
Table 7.10 lists the advantages and disadvantages of SSCEs.
Table 7.10 The advantages and disadvantages of using the silver–silver chloride electrode (SSCE)
Advantages of the SSCE
Cost The SSCE is easy and extremely cheap to make
Stability The SSCE has the smallest temperature voltage coefficient
of any common reference electrode
Size An SSCE can be as large or small as desired; it can even be
microscopic if the silver is thin enough
Disadvantages of the SSCE
Photochemical stability The layer of AgCl must be remade often
−
Contamination The solid AgCl loses Cl ions during photolytic breakdown