Page 367 - Physical chemistry understanding our chemical world
P. 367
334 ELECTROCHEMISTRY
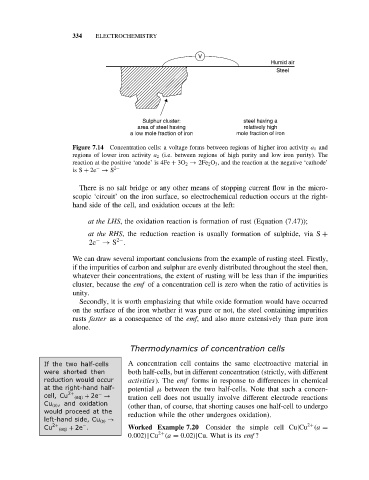
V
Humid air
Steel
Sulphur cluster: steel having a
area of steel having relatively high
a low mole fraction of iron mole fraction of iron
Figure 7.14 Concentration cells: a voltage forms between regions of higher iron activity a 1 and
regions of lower iron activity a 2 (i.e. between regions of high purity and low iron purity). The
reaction at the positive ‘anode’ is 4Fe + 3O 2 → 2Fe 2 O 3 , and the reaction at the negative ‘cathode’
is S + 2e → S 2−
−
There is no salt bridge or any other means of stopping current flow in the micro-
scopic ‘circuit’ on the iron surface, so electrochemical reduction occurs at the right-
hand side of the cell, and oxidation occurs at the left:
at the LHS, the oxidation reaction is formation of rust (Equation (7.47));
at the RHS, the reduction reaction is usually formation of sulphide, via S +
−
2−
2e → S .
We can draw several important conclusions from the example of rusting steel. Firstly,
if the impurities of carbon and sulphur are evenly distributed throughout the steel then,
whatever their concentrations, the extent of rusting will be less than if the impurities
cluster, because the emf of a concentration cell is zero when the ratio of activities is
unity.
Secondly, it is worth emphasizing that while oxide formation would have occurred
on the surface of the iron whether it was pure or not, the steel containing impurities
rusts faster as a consequence of the emf, and also more extensively than pure iron
alone.
Thermodynamics of concentration cells
If the two half-cells A concentration cell contains the same electroactive material in
were shorted then both half-cells, but in different concentration (strictly, with different
reduction would occur activities). The emf forms in response to differences in chemical
at the right-hand half- potential µ between the two half-cells. Note that such a concen-
cell, Cu 2+ (aq) + 2e → tration cell does not usually involve different electrode reactions
−
Cu (s) , and oxidation (other than, of course, that shorting causes one half-cell to undergo
would proceed at the
reduction while the other undergoes oxidation).
left-hand side, Cu (s) →
2+
−
Cu 2+ (aq) + 2e . Worked Example 7.20 Consider the simple cell Cu|Cu (a =
2+
0.002)||Cu (a = 0.02)|Cu. What is its emf ?