Page 262 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 262
234 Polymer-based Nanocomposites for Energy and Environmental Applications
subjected to hydrogen storage application. On comparing with the hydrogen storage
capacity of pristine nanotubes (0.019 wt%) and PANI (0.05 wt%), the optimum
nanocomposites show an improved capacity of 0.78 wt% with excellent reversibility.
This can be accredited to the chemisorption nature of hydrogen uptake, which was
improved by the sorption sites fashioned through the milling process of PANI with
the nanotubes. Further, the controlled lumen size is more appropriate for a supreme
hydrogen adsorption during inserting PANI chains into the nanotubes [57].
Jurczyk et al. have investigated the hydrogen storage capacity of PANI with dif-
ferent nanofillers and their effect on storage capacity. The hydrogen sorption prop-
erties of PANI with tin oxide (SnO 2 ) were investigated over the temperature range of
298–398 K. The pristine PANI shows a hydrogen sorption capacity of 0.35 wt% at
398 K and 6 MPa, whereas the nanocomposite PANI-SnO 2 did not show a noticeable
enhancement in the hydrogen sorption capacity compared with pristine PANI [20].
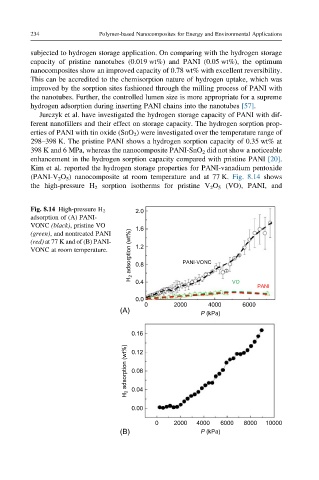
Kim et al. reported the hydrogen storage properties for PANI-vanadium pentoxide
(PANI-V 2 O 5 ) nanocomposite at room temperature and at 77 K. Fig. 8.14 shows
the high-pressure H 2 sorption isotherms for pristine V 2 O 5 (VO), PANI, and
Fig. 8.14 High-pressure H 2 2.0
adsorption of (A) PANI-
VONC (black), pristine VO 1.6
H 2 adsorption (wt%) 0.8 PANI-VONC
(green), and nontreated PANI
(red) at 77 K and of (B) PANI-
VONC at room temperature. 1.2
0.4 VO
PANI
0.0
0 2000 4000 6000
(A)
P (kPa)
0.16
H 2 adsorption (wt%) 0.08
0.12
0.04
0.00
0 2000 4000 6000 8000 10000
(B) P (kPa)