Page 257 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 257
Polyaniline-based nanocomposites for hydrogen storage 229
typical dimensions of around 200 75 50 nm (height width thickness) at the
mouth of the tube. The protrusions were noticed on both the outside and inside sur-
faces of the tubes (Fig. 8.8C). Related with ARP-CTs-30, the tube morphologies of
ARP-CTs-45 and ARP-CTs-60 become round shape on the outside and square shape
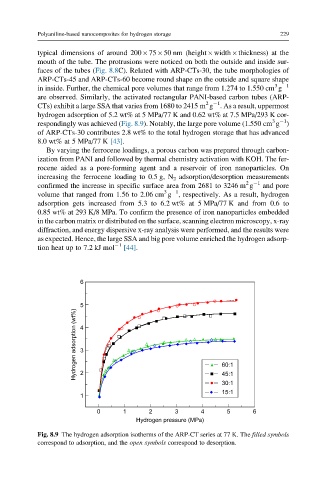
3 1
in inside. Further, the chemical pore volumes that range from 1.274 to 1.550 cm g
are observed. Similarly, the activated rectangular PANI-based carbon tubes (ARP-
2 1
CTs) exhibit a large SSA that varies from 1680 to 2415 m g . As a result, uppermost
hydrogen adsorption of 5.2 wt% at 5 MPa/77 K and 0.62 wt% at 7.5 MPa/293 K cor-
3 1
respondingly was achieved (Fig. 8.9). Notably, the large pore volume (1.550 cm g )
of ARP-CTs-30 contributes 2.8 wt% to the total hydrogen storage that has advanced
8.0 wt% at 5 MPa/77 K [43].
By varying the ferrocene loadings, a porous carbon was prepared through carbon-
ization from PANI and followed by thermal chemistry activation with KOH. The fer-
rocene aided as a pore-forming agent and a reservoir of iron nanoparticles. On
increasing the ferrocene loading to 0.5 g, N 2 adsorption/desorption measurements
2 1
confirmed the increase in specific surface area from 2681 to 3246 m g and pore
3 1
volume that ranged from 1.56 to 2.06 cm g , respectively. As a result, hydrogen
adsorption gets increased from 5.3 to 6.2 wt% at 5 MPa/77 K and from 0.6 to
0.85 wt% at 293 K/8 MPa. To confirm the presence of iron nanoparticles embedded
in the carbon matrix or distributed on the surface, scanning electron microscopy, x-ray
diffraction, and energy dispersive x-ray analysis were performed, and the results were
as expected. Hence, the large SSA and big pore volume enriched the hydrogen adsorp-
tion heat up to 7.2 kJ mol 1 [44].
6
5
Hydrogen adsorption (wt%) 4
3
60:1
2
45:1
30:1
15:1
1
0 1 2 3 4 5 6
Hydrogen pressure (MPa)
Fig. 8.9 The hydrogen adsorption isotherms of the ARP-CT series at 77 K. The filled symbols
correspond to adsorption, and the open symbols correspond to desorption.