Page 253 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 253
Polyaniline-based nanocomposites for hydrogen storage 225
criteria for hydrogen uptake. Intrinsic microporosity (microporosity without
possessing a network of covalent bonds) is one of the methods to create pores in poly-
mer systems [13,33]. Moreover, pores in polymers with intrinsic microporosity
(PIMs) are designed due to the ineffective packing of rigid polymer subunits. Conven-
tionally, PIMs are designed by connecting two or more rigid moieties through an
arrangement that forces the nonplanarity in the final molecule. Also, PIM porosity
is independent of the processing history [6,13,31–33]. Usually, surface areas of PIMs
2 1
could be in the range of 300–1500 m g . For instance, trip-PIM based on a triptycene
monomer takes up to 2.7 wt% H 2 by mass at 10 bar and 77 K or 3.04 wt% at 15 bar
and 77 K. By considering the uncertain extrapolation of H 2 sorption capacity versus
2 1
the PIM surface area, it was proposed that a PIM with surface area of 2400 m g
could adsorb >6 wt% H 2 by mass at 15 bar and 77 K. Hence, it is necessary to find
the appropriate methods to synthesize the high-surface-area materials [6].
Due to the porous nature of the hypercross-linked polymers (HCPs), it is more
suitable for hydrogen storage [15,36]. Germain et al. synthesized hypercross-linked
2 1
PANI with permanent pore structure and specific surface area (SSA) of 632 m g .
Similarly, short cross-links could also be prepared possible using paraformaldehyde
and diiodomethane with the high-surface area. It is to be noted that during the prep-
aration of PANI, increasing the monomer concentration lowers the surface area. The
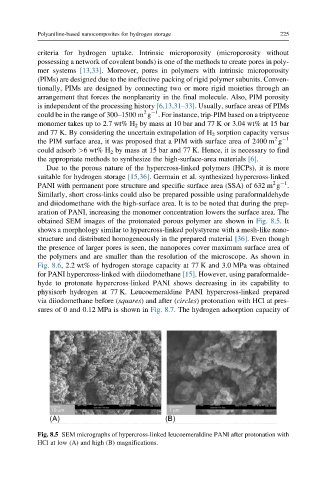
obtained SEM images of the protonated porous polymer are shown in Fig. 8.5.It
shows a morphology similar to hypercross-linked polystyrene with a mesh-like nano-
structure and distributed homogeneously in the prepared material [36]. Even though
the presence of larger pores is seen, the nanopores cover maximum surface area of
the polymers and are smaller than the resolution of the microscope. As shown in
Fig. 8.6, 2.2 wt% of hydrogen storage capacity at 77 K and 3.0 MPa was obtained
for PANI hypercross-linked with diiodomethane [15]. However, using paraformalde-
hyde to protonate hypercross-linked PANI shows decreasing in its capability to
physisorb hydrogen at 77 K. Leucoemeraldine PANI hypercross-linked prepared
via diiodomethane before (squares) and after (circles) protonation with HCl at pres-
sures of 0 and 0.12 MPa is shown in Fig. 8.7. The hydrogen adsorption capacity of
Fig. 8.5 SEM micrographs of hypercross-linked leucoemeraldine PANI after protonation with
HCl at low (A) and high (B) magnifications.