Page 248 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 248
220 Polymer-based Nanocomposites for Energy and Environmental Applications
adsorption material by means of weak van der Waals interactions, where hydrogen
keeps its molecular form and no chemical reactions like dissociation occurs.
In this context, intrinsically conducting polymers (ICPs) such as polyaniline
(PANI), polypyrrole (PPY), polythiophene, poly(paraphenylenevinylene), and
poly(paraphenylene) have been employed as hydrogen storage materials due to its
thermal, unique electronic properties and the presence of physisorption and chemi-
sorption sites [13–15]. In addition, these polymers tend to have numerous hydrogen
atoms that favor the creation of fragile secondary bonds between hydrogen that is in
the portion of the polymers and the hydrogen that is expected to be stored [16]. Hence,
ICPs play a major role in hydrogen storage. Among them, PANI shows exceptional
thermal, electric, and environmental behaviors. Specifically, porous and nonporous
PANI and their nanocomposites have been generally used in hydrogen storage appli-
cations owing to its physisorption and chemisorption reactive sites for reversible
hydrogen storage [17]. The present chapter deals with various structures of PANI
including nonporous and nanoporous, and its nanocomposites for hydrogen storage
applications are discussed. Importantly, the hydrogen storage mechanism based on
the unique electronic properties of PANI due to π electron in backbone structure is
also highlighted.
8.2 Polyaniline (PANI) for hydrogen storage
Among the ICPs, PANI has a lot of advantages due to simple synthesis nature, low-
cost monomer, good process ability, electric conductivity, and highest thermal stabil-
ity [16]. Also it is known that it can exist in three different oxidation states including
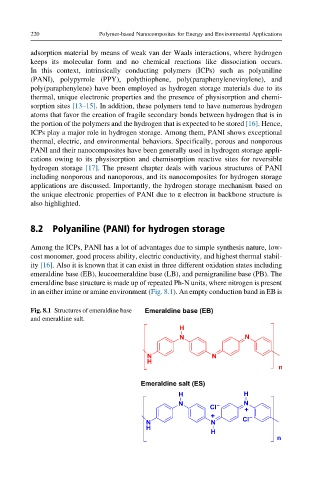
emeraldine base (EB), leucoemeraldine base (LB), and pernigraniline base (PB). The
emeraldine base structure is made up of repeated Ph-N units, where nitrogen is present
in an either imine or amine environment (Fig. 8.1). An empty conduction band in EB is
Fig. 8.1 Structures of emeraldine base Emeraldine base (EB)
and emeraldine salt.
Emeraldine salt (ES)