Page 249 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 249
Polyaniline-based nanocomposites for hydrogen storage 221
responsible for electrically insulating nature of the material, whereas on doping with
strong acid, it protonates both iminic nitrogens to provide emeraldine salt (ES)
(Fig. 8.1). Hence, ES exhibits electrically conducting nature due to its half-filled
polaron band. This favors ES to be used in different potential applications [14,16].
Due to the electronic properties, the delocalization of charges along with the polymer
backbone makes PANI as an appropriate contender for hydrogen storage applications.
Various structures and composites of PANI including nonporous and porous PANI
[18], activated porous PANI [19], PANI-metal composites [20], PANI-carbon com-
posites [21], and PANI-metal oxide composites [22] are focused as hydrogen storage
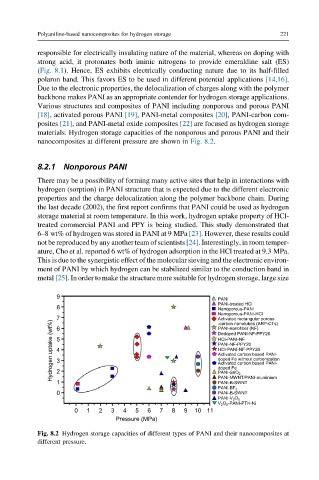
materials. Hydrogen storage capacities of the nonporous and porous PANI and their
nanocomposites at different pressure are shown in Fig. 8.2.
8.2.1 Nonporous PANI
There may be a possibility of forming many active sites that help in interactions with
hydrogen (sorption) in PANI structure that is expected due to the different electronic
properties and the charge delocalization along the polymer backbone chain. During
the last decade (2002), the first report confirms that PANI could be used as hydrogen
storage material at room temperature. In this work, hydrogen uptake property of HCl-
treated commercial PANI and PPY is being studied. This study demonstrated that
6–8 wt% of hydrogen was stored in PANI at 9 MPa [23]. However, these results could
not be reproduced by any another team of scientists [24]. Interestingly, in room temper-
ature, Cho et al. reported 6 wt% of hydrogen adsorption in the HCl treated at 9.3 MPa.
This is due to the synergistic effect of the molecular sieving and the electronic environ-
ment of PANI by which hydrogen can be stabilized similar to the conduction band in
metal [25]. In order to make the structure more suitable for hydrogen storage, large size
9
PANI
PANI-treated HCI
8 Nanoporous-PANI
Nanoporous-PANI-HCI
7 6 Activated rectangular porous
Hydrogen uptake (wt%) 5 4 3 HCI-PANI-NF
-carbon nanotubes (ARP-CTs)
PANI-nanofiber (NF)
Dedoped PANI-NF-PPY20
PANI-NF-PPY20
HCI-PANI-NF-PPY20
Activated carbon based PANI-
doped Fe without carbonization
Activated carbon based PANI-
PANI-SnO 2
PANI-MWNT/PANI-aluminium
1 2 doped Fe
PANI-B-SWNT
PANI-BF 3
0 PANI-B-SWNT
PANI-V 2 O 5
V 2 O 5 -PANI-PTH-Ni
0 1 2 3 4 5 6 7 8 9 10 11
Pressure (MPa)
Fig. 8.2 Hydrogen storage capacities of different types of PANI and their nanocomposites at
different pressure.